Better Together: Functional Heterobimetallic Macrocyclic and Cage-like Assemblies
- PMID: 32297380
- PMCID: PMC7693062
- DOI: 10.1002/chem.202001602
Better Together: Functional Heterobimetallic Macrocyclic and Cage-like Assemblies
Abstract
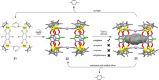
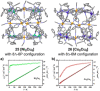
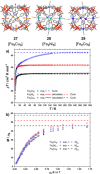
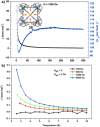
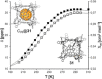
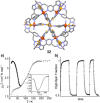
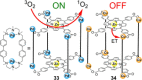
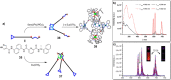
Metallosupramolecular chemistry has attracted the interest of generations of researches due to the versatile properties and functionalities of oligonuclear coordination complexes. Quite a number of different discrete cages were investigated, mostly consisting of only one type of ligand and one type of metal cation. Looking for ever more complex structures, heterobimetallic complexes became more and more attractive, as they give access to new structural motifs and functions. In the last years substantial success has been made in the design and synthesis of cages consisting of more than one type of metal cations, and a rapidly growing number of functional materials has appeared in the literature. This Minireview describes recent developments in the field of discrete heterometallic macrocycles and cages focusing on functional materials that have been used as host-systems or as magnetic, photo-active, redox-active, and even catalytically active materials.
Keywords: functional materials; heterobimetallic complexes; metallosupramolecular cages; supramolecular catalysts; supramolecular chemistry.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
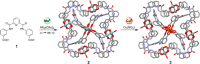

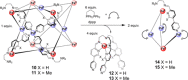
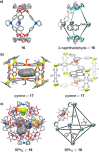
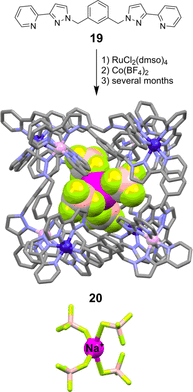

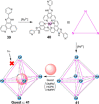
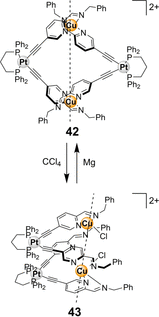
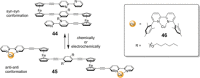
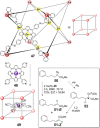
References
-
- Caulder D. L., Raymond K. N., J. Chem. Soc. Dalton Trans. 1999, 1185–1200.
-
- Olenyuk B., Fechtenkötter A., Stang P. J., J. Chem. Soc. Dalton Trans. 1998, 1707–1728.
-
- Fujita M., Chem. Soc. Rev. 1998, 27, 417.
-
- Smulders M. M. J., Riddell I. A., Browne C., Nitschke J. R., Chem. Soc. Rev. 2013, 42, 1728–1754. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

