Functional characterization of the antiepileptic drug candidate, padsevonil, on GABAA receptors
- PMID: 32297665
- PMCID: PMC7383892
- DOI: 10.1111/epi.16497
Functional characterization of the antiepileptic drug candidate, padsevonil, on GABAA receptors
Abstract
Objective: The antiepileptic drug candidate, padsevonil, is the first in a novel class of drugs designed to interact with both presynaptic and postsynaptic therapeutic targets: synaptic vesicle 2 proteins and γ-aminobutyric acid type A receptors (GABAA Rs), respectively. Functional aspects of padsevonil at the postsynaptic target, GABAA Rs, were characterized in experiments reported here.
Methods: The effect of padsevonil on GABA-mediated Cl- currents was determined by patch clamp on recombinant human GABAA Rs (α1β2γ2) stably expressed in a CHO-K1 cell line and on native GABAA Rs in cultured rat primary cortical neurons. Padsevonil selectivity for GABAA R subtypes was evaluated using a two-electrode voltage clamp on recombinant human GABAA Rs (α1-5/β2/γ2) in Xenopus oocytes.
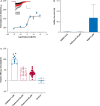
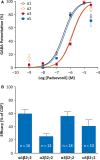
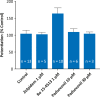
Results: In recombinant GABAA Rs, padsevonil did not evoke Cl- currents in the absence of the agonist GABA. However, when co-administered with GABA at effective concentration (EC)20 , padsevonil potentiated GABA responses by 167% (EC50 138 nmol/L) and demonstrated a relative efficacy of 41% compared with zolpidem, a reference benzodiazepine site agonist. Similarly, padsevonil demonstrated GABA-potentiating activity at native GABAA Rs (EC50 208 nmol/L) in cultured rat cortical neurons. Padsevonil also potentiated GABA (EC20 ) responses in GABAA Rs expressed in oocytes, with higher potency at α1- and α5-containing receptors (EC50 295 and 281 nmol/L) than at α2- and α3-containing receptors (EC50 1737 and 2089 nmol/L). Compared with chlordiazepoxide-a nonselective, full GABAA R agonist-the relative efficacy of padsevonil was 60% for α1β2γ2, 26% for α2β2γ2, 56% for α3β2γ2, and 41% for α5β2γ2; no activity was observed at benzodiazepine-insensitive α4β2γ2 receptors.
Significance: Results of functional investigations on recombinant and native neuronal GABAA Rs show that padsevonil acts as a positive allosteric modulator of these receptors, with a partial agonist profile at the benzodiazepine site. These properties may confer better tolerability and lower potential for tolerance development compared with classic benzodiazepines currently used in the clinic.
Keywords: SV2 proteins; benzodiazepine; drug-resistant epilepsy; patch clamp.
© 2020 UCB Biopharma SRL. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Conflict of interest statement
All authors were employees of UCB Pharma at the time of research. MW has now retired, RMK is currently employed by OncoArendi Therapeutics SA, Warsaw, Poland, and PG by Pi life sciences consultancy, Ghent, Belgium. The authors confirm having read the Journal's position on ethical publication and affirm that this report is consistent with Journal guidelines.
Figures

References
-
- Kaminski RM, Matagne A, Patsalos PN, Klitgaard H. Benefit of combination therapy in epilepsy: a review of the preclinical evidence with levetiracetam. Epilepsia. 2009;50:387–97. - PubMed
-
- Wood M, Daniels V, Provins L, Wolff C, Kaminski RM, Gillard M. Pharmacological profile of the antiepileptic drug candidate padsevonil – interactions with synaptic vesicle 2 proteins and GABAA receptors. J Pharmacol Exp Ther. 2020;372:1–10. - PubMed
-
- Wood MD, Gillard M. Evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia. 2017;58:255–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

