Small proline-rich repeat 3 is a novel coordinator of PDGFRβ and integrin β1 crosstalk to augment proliferation and matrix synthesis by cardiac fibroblasts
- PMID: 32297675
- PMCID: PMC7302973
- DOI: 10.1096/fj.201902815R
Small proline-rich repeat 3 is a novel coordinator of PDGFRβ and integrin β1 crosstalk to augment proliferation and matrix synthesis by cardiac fibroblasts
Abstract
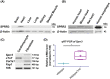
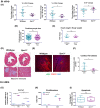
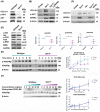
Nearly 6 million Americans suffer from heart failure. Increased fibrosis contributes to functional decline of the heart that leads to heart failure. Previously, we identified a mechanosensitive protein, small proline-rich repeat 3 (SPRR3), in vascular smooth muscle cells of atheromas. In this study, we demonstrate SPRR3 expression in cardiac fibroblasts which is induced in activated fibroblasts following pressure-induced heart failure. Sprr3 deletion in mice showed preserved cardiac function and reduced interstitial fibrosis in vivo and reduced fibroblast proliferation and collagen expression in vitro. SPRR3 loss resulted in reduced activation of Akt, FAK, ERK, and p38 signaling pathways, which are coordinately regulated by integrins and growth factors. SPRR3 deletion did not impede integrin-associated functions including cell adhesion, migration, or contraction. SPRR3 loss resulted in reduced activation of PDGFRβ in fibroblasts. This was not due to the reduced PDGFRβ expression levels or decreased binding of the PDGF ligand to PDGFRβ. SPRR3 facilitated the association of integrin β1 with PDGFRβ and subsequently fibroblast proliferation, suggesting a role in PDGFRβ-Integrin synergy. We postulate that SPRR3 may function as a conduit for the coordinated activation of PDGFRβ by integrin β1, leading to augmentation of fibroblast proliferation and matrix synthesis downstream of biomechanical and growth factor signals.
Keywords: collagen; fibrosis; heart failure; pressure overload; transverse aortic constriction.
© 2020 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40‐51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

