Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer
- PMID: 32297899
- PMCID: PMC7163782
- DOI: 10.1001/jamaoncol.2020.0726
Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer
Abstract
Importance: Limited information is available on the safety of a rechallenge with an immune checkpoint inhibitor (ICI) after an immune-related adverse event (irAE).
Objective: To identify the recurrence rate of the same irAE that prompted discontinuation of ICI therapy after an ICI rechallenge in patients with cancer and to identify the clinical features associated with such recurrences.
Design, setting, and participants: This observational, cross-sectional, pharmacovigilance cohort study examined individual case safety reports from the World Health Organization database VigiBase, which contains case reports from more than 130 countries. Case reports were extracted from database inception (1967) to September 1, 2019. All consecutive ICI cases with at least 1 associated irAE were included.
Main outcomes and measures: The primary outcome was the rate of recurrence of the initial irAE after an ICI rechallenge. Secondary outcomes included the factors associated with the recurrence after a rechallenge among informative rechallenges, the recurrence rate according to the ICI regimen (anti-programmed cell death 1 or anti-programmed cell death ligand 1 monotherapy, anti-cytotoxic T-lymphocyte antigen-4 monotherapy, or combination therapy), and the rate of occurrence of a different irAE after a rechallenge.
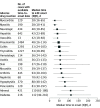
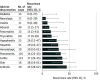
Results: A total of 24 079 irAE cases associated with at least 1 ICI were identified. Among the irAEs, 452 of 6123 irAEs associated with ICI rechallenges (7.4%) were informative rechallenges. One hundred thirty recurrences (28.8%; 95% CI, 24.8-33.1) of the initial irAE were observed. In a rechallenge, colitis (reporting odds ratio [OR], 1.77; 95% CI, 1.14-2.75; P = .01), hepatitis (reporting OR, 3.38; 95% CI, 1.31-8.74; P = .01), and pneumonitis (reporting OR, 2.26; 95% CI, 1.18-4.32; P = .01) were associated with a higher recurrence rate, whereas adrenal events were associated with a lower recurrence rate (reporting OR, 0.33; 95% CI, 0.13-0.86; P = .03) compared with other irAEs.
Conclusions and relevance: This cohort study found a 28.8% recurrence rate of the same irAE associated with the discontinuation of ICI therapy after a rechallenge with the same ICI. Resuming ICI therapy could be considered for select patients, with appropriate monitoring and use of standard treatment algorithms to identify and treat toxic effects.
Conflict of interest statement
Figures
Comment in
-
Recurrence of Immune-Related Adverse Events After Immune Checkpoint Inhibitor Rechallenge-Reply.JAMA Oncol. 2020 Nov 1;6(11):1814-1815. doi: 10.1001/jamaoncol.2020.3960. JAMA Oncol. 2020. PMID: 32970107 No abstract available.
-
Recurrence of Immune-Related Adverse Events After Immune Checkpoint Inhibitor Rechallenge.JAMA Oncol. 2020 Nov 1;6(11):1813. doi: 10.1001/jamaoncol.2020.3946. JAMA Oncol. 2020. PMID: 32970125 No abstract available.
-
Recurrence of Immune-Related Adverse Events After Immune Checkpoint Inhibitor Rechallenge.JAMA Oncol. 2020 Nov 1;6(11):1813-1814. doi: 10.1001/jamaoncol.2020.3955. JAMA Oncol. 2020. PMID: 32970130 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

