Interventions for fatigue in inflammatory bowel disease
- PMID: 32297974
- PMCID: PMC7161727
- DOI: 10.1002/14651858.CD012005.pub2
Interventions for fatigue in inflammatory bowel disease
Abstract
Background: Inflammatory bowel disease (IBD) is an umbrella term used to describe a group of chronic, progressive inflammatory disorders of the digestive tract. Crohn's disease and ulcerative colitis are the two main types. Fatigue is a common, debilitating and burdensome symptom experienced by individuals with IBD. The subjective, complex nature of fatigue can often hamper its management. The efficacy and safety of pharmacological or non-pharmacological treatments for fatigue in IBD is not yet established through systematic review of studies.
Objectives: To assess the efficacy and safety of pharmacological and non-pharmacological interventions for managing fatigue in IBD compared to no treatment, placebo or active comparator.
Search methods: A systematic search of the databases Embase, MEDLINE, Cochrane Library, CINAHL, PsycINFO was undertaken from inception to July 2018. A top-up search was run in October 2019. We also searched the Cochrane IBD Group Specialized Register, the Cochrane Central Register of Controlled Trials, ongoing trials and research registers, conference abstracts and reference lists for potentially eligible studies.
Selection criteria: Randomised controlled trials of pharmacological and non-pharmacological interventions in children or adults with IBD, where fatigue was assessed as a primary or secondary outcome using a generic or disease-specific fatigue measure, a subscale of a larger quality of life scale or as a single-item measure, were included.
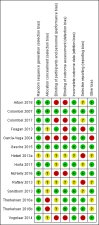
Data collection and analysis: Two authors independently screened search results and four authors extracted and assessed bias independently using the Cochrane 'Risk of bias' tool. The primary outcome was fatigue and the secondary outcomes included quality of life, adverse events (AEs), serious AEs and withdrawal due to AEs. Standard methodological procedures were used.
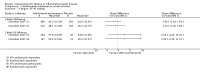
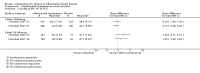
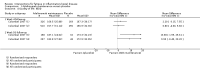
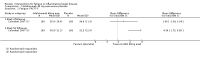
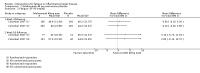
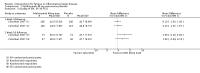
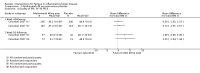
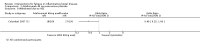
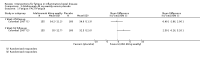
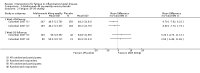
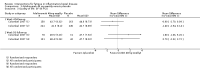
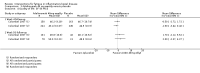
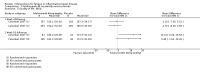
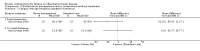
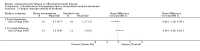
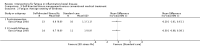
Main results: We included 14 studies (3741 participants): nine trials of pharmacological interventions and five trials of non-pharmacological interventions. Thirty ongoing studies were identified, and five studies are awaiting classification. Data on fatigue were available from nine trials (1344 participants). In only four trials was managing fatigue the primary intention of the intervention (electroacupuncture, physical activity advice, cognitive behavioural therapy and solution-focused therapy). Electroacupuncture Fatigue was measured with Functional Assessment of Chronic Illness Therapy - Fatigue (FACIT-F) (scores range from 0 to 52). The FACIT-F score at week eight was 8.00 points higher (better) in participants receiving electroacupuncture compared with no treatment (mean difference (MD) 8.00, 95% CI 6.45 to 9.55; 1 RCT; 27 participants; low-certainty evidence). Results at week 16 could not be calculated. FACIT-F scores were also higher with electroacupuncture compared to sham electroacupuncture at week eight (MD 5.10, 95% CI 3.49 to 6.71; 1 RCT; 30 participants; low-certainty evidence) but not at week 16 (MD 2.60, 95% CI 0.74 to 4.46; 1 RCT; 30 participants; low-certainty evidence). No adverse events were reported, except for one adverse event in the sham electroacupuncture group. Cognitive behavioural therapy (CBT) and solution-focused therapy Compared with a fatigue information leaflet, the effects of CBT on fatigue are very uncertain (Inflammatory Bowel Disease-Fatigue (IBD-F) section I: MD -2.16, 95% CI -6.13 to 1.81; IBD-F section II: MD -21.62, 95% CI -45.02 to 1.78; 1 RCT, 18 participants, very low-certainty evidence). The efficacy of solution-focused therapy on fatigue is also very uncertain, because standard summary data were not reported (1 RCT, 98 participants). Physical activity advice One 2 x 2 factorial trial (45 participants) found physical activity advice may reduce fatigue but the evidence is very uncertain. At week 12, compared to a control group receiving no physical activity advice plus omega 3 capsules, FACIT-F scores were higher (better) in the physical activity advice plus omega 3 group (FACIT-F MD 6.40, 95% CI -1.80 to 14.60, very low-certainty evidence) and the physical activity advice plus placebo group (FACIT-F MD 9.00, 95% CI 1.64 to 16.36, very low-certainty evidence). Adverse events were predominantly gastrointestinal and similar across physical activity groups, although more adverse events were reported in the no physical activity advice plus omega 3 group. Pharmacological interventions Compared with placebo, adalimumab 40 mg, administered every other week ('eow') (only for those known to respond to adalimumab induction therapy), may reduce fatigue in patients with moderately-to-severely active Crohn's disease, but the evidence is very uncertain (FACIT-F MD 4.30, 95% CI 1.75 to 6.85; very low-certainty evidence). The adalimumab 40 mg eow group was less likely to experience serious adverse events (OR 0.56, 95% CI 0.33 to 0.96; 521 participants; moderate-certainty evidence) and withdrawal due to adverse events (OR 0.48, 95%CI 0.26 to 0.87; 521 participants; moderate-certainty evidence). Ferric maltol may result in a slight increase in fatigue, with better SF-36 vitality scores reported in the placebo group compared to the treatment group following 12 weeks of treatment (MD -9.31, 95% CI -17.15 to -1.47; 118 participants; low-certainty evidence). There may be little or no difference in adverse events (OR 0.55, 95% CI 0.26 to 1.18; 120 participants; low-certainty evidence) AUTHORS' CONCLUSIONS: The effects of interventions for the management of fatigue in IBD are uncertain. No firm conclusions regarding the efficacy and safety of interventions can be drawn. Further high-quality studies, with a larger number of participants, are required to assess the potential benefits and harms of therapies. Future studies should assess interventions specifically designed for fatigue management, targeted at selected IBD populations, and measure fatigue as the primary outcome.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Dawn Farrell: This paper presents independent research funded by the Health Research Board of Ireland under the Cochrane Fellowship programme (Reference Number CFT‐2014‐887). Infrastructure support was provided from the host institution, University College Cork, to conduct this review.
Micol Artom: This paper presents independent research funded by the UK National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Reference Number RP‐PG‐0216‐20001). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
Wladyslawa Czuber‐Dochan: Serves as a member of Scientific Committee for Crohn's and Colitis UK, and Nurse‐European Crohn's and Colitis Organisation. She has also received speaker fees from Pfizer. This paper presents independent research funded by the UK National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Reference Number RP‐PG‐0216‐20001). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
Lars‐Petter Jelsness‐Jørgensen has received unrestricted research grants from Ferring Pharmaceuticals and Tillots Pharma. and has also acted as consultant and speaker for Abbvie. All of these activities are outside the submitted work.
Christine Norton: Tillotts, Takeda, AbbVie, Ferring (lecture fees) (outside submitted work). This paper presents independent research funded by the UK National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Reference Number RP‐PG‐0216‐20001). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
Eileen Savage: None known.
Figures
















































Update of
References
References to studies included in this review
Artom 2018 {published and unpublished data}
-
- ISRCTN17917944. Management of inflammatory bowel disease‐fatigue: a pilot cognitive‐behavioural therapy interventional study. http://www.isrctn.com/ISRCTN17917944 (first received 2 September 2016).
Colombel 2007 {published data only}
-
- Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 2007;132(1):52‐65. - PubMed
-
- Loftus EV, Feagan BG, Colombel J‐F, Rubin DT, Wu EQ, Yu AP, et al. Effects of adalimumab maintenance therapy on health‐related quality of life of patients with Crohn’s disease: patient‐reported outcomes of the CHARM Trial. American Journal of Gastroenterology 2008;103(7):3132‐41. - PubMed
-
- Rubin D, Rutgeerts P, Colombel J, Wu E, Yu A, Chao J, et al. Adalimumab maintenance therapy and health related quality of life in TNT‐antagonist‐naïve patients with Crohn’s disease. Journal of the Canadian Association of Gastroenterology 2009;23(1):S118.
Colombel 2017 {published data only}
-
- Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017;390(10114):2779‐89. - PubMed
-
- NCT01235689. An open‐label, multicenter, efficacy and safety study to evaluate two treatment algorithms in subjects with moderate to severe Crohn's Disease. clinicaltrials.gov/ct2/show/NCT01235689 (first received 5 November 2010).
-
- Panaccione R, Colombel JF, Bossuyt P, Baert F, Vanasek T, Danalioglu A, et al. DOP071 Tight control with adalimumab‐based treatment is associated with improved quality of life outcomes in patients with moderate to severely active Crohn’s disease: data from CALM. Journal of Crohn's and Colitis 2018;12:S078‐9.
Feagan 2013 {published data only}
-
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699‐710. - PubMed
-
- NCT00783718. A phase 3, randomized, placebo‐controlled, blinded, multicenter study of the induction and maintenance of clinical response and remission by MLN0002 in patients with moderate to severe ulcerative colitis. clinicaltrials.gov/ct2/show/NCT00783718 (first received 2 November 2008).
-
- Rubin DT, Tudor D, Khalid JM, Patel H. P570 Improvements in subcomponents of the inflammatory bowel disease questionnaire in patients treated with vedolizumab: results from GEMINI trial data. Journal of Crohn's and Colitis 2018;12(Supplement 1):S394‐5.
García‐Vega 2004 {published data only}
-
- García‐Vega E, Fernandez‐Rodriguez C. A stress management programme for Crohn’s disease. Behaviour Research and Therapy 2004;42(4):367‐83. - PubMed
Gasche 2015 {published data only}
Hetzel 2013a {published data only}
-
- Hetzel D, Barish C, Dahl N, Bernard K, Lau G, Strauss W. Efficacy and safety of intravenous Ferunoxytol for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2013;19(Supplement 1):S81‐2.
-
- Hetzel D, Ford D, Dahl N, Strauss W. Intravenous (IV) ferumoxytol (FER) for the treatment of iron deficiency anaemia (IDA) in patients with inflammatory bowel disease (IBD): efficacy, safety and health‐related quality of life (HRQOL). Journal of Crohn's and Colitis 2014;8:S248‐9.
Horta 2017 {published and unpublished data}
-
- Horta D, Sanchez‐Lloansi M, Lira A, Villoria A, Teggiachi M, Garcia D, et al. A prospective randomised study: electroacupuncture vs. sham procedure for the treatment of fatigue in patients with quiescent inflammatory bowel disease. United European Gastroenterology Journal 2017;5(Supplement 1):A293‐4.
McNelly 2016 {published data only}
-
- McNelly AS, Nathan I, Monti M, Grimble GK, Norton C, Bredin F, et al. Inflammatory bowel disease and fatigue: the effect of physical activity and/or omega‐3 supplementation. Journal of Crohn's and Colitis 2016;10:S370‐1.
-
- McNelly AS, Nathan I, Monti M, Grimble GK, Norton C, Bredin F, et al. The effect of increasing physical activity and/or omega‐3 supplementation on fatigue in inflammatory bowel disease. Gastrointestinal Nursing 2016;14(8):39‐50.
Raftery 2013 {published data only}
-
- NCT01792388. Vitamin D and its effects on inflammation and intestinal permeability in Crohn's disease in remission. clinicaltrials.gov/ct2/show/NCT01792388 (first received 15 February 2013).
-
- Raftery TC, Healy M, Cox G, McNamara D, O’ Sullivan M. Vitamin D supplementation improves muscles strength, fatigue and quality of life in patients with Crohn’s disease in remission: results of a randomised double‐blind placebo‐controlled study. Gastroenterology 2013;144(5):S227.
Sandborn 2013 {published data only}
-
- NCT00783692. A phase 3, randomized, placebo‐controlled, blinded, multicenter study of the induction and maintenance of clinical response and remission by vedolizumab (MLN0002) in patients with moderate to severe Crohn's Disease. clinicaltrials.gov/ct2/show/study/NCT00783692 (first received 2 November 2008).
-
- Rubin DT, Tudor D, Khalid JM, Patel H. P570 Improvements in subcomponents of the inflammatory bowel disease questionnaire in patients treated with vedolizumab: results from GEMINI trial data. Journal of Crohn's and Colitis 2018;12(Supplement 1):S394‐5.
-
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. New England Journal of Medicine 2013;369(8):711‐21. - PubMed
Therkelsen 2016a {published data only}
-
- Therkelsen SP, Hetland G, Lyberg T, Lygren I, Johnson E. Effect of a medicinal agaricus blazei murill‐based mushroom extract, AndoSan™, on symptoms, fatigue and quality of life in patients with ulcerative colitis in a randomized single‐blinded placebo controlled study. PlOS One 2016;11(3):e0150191. - PMC - PubMed
Therkelsen 2016b {published data only}
-
- Therkelsen SP, Hetland G, Lyberg T, Lygren I, Johnson E. Effect of the medicinal agaricus blazei murill‐based mushroom extract, AndoSanTM, on symptoms, fatigue and quality of life in patients with Crohn’s Disease in a randomized single‐blinded placebo controlled study. PLOS One 2016;11(7):e0159288. - PMC - PubMed
Vogelaar 2014 {published data only}
-
- Vogelaar L, Van't Spijker A, Timman R, Tilburg A, Bac D, Kuipers EJ, et al. Fatigue in IBD patients decreases with psychotherapy: results of a randomized controlled trial. Journal of Crohn's and Colitis 2013;7:S211‐12.
-
- Vogelaar L, Van’t Spijker A, Timman R, Tilburg AJP, Bac D, Vogelaar T, et al. Fatigue management in patients with IBD: a randomised controlled trial. Gut 2014;63:911‐8. - PubMed
References to studies excluded from this review
Boye 2011 {published data only}
-
- Boye B, Lundin KE, Jantschek G, Leganger S, Mokleby K, Tangen T, et al. INSPIRE study: does stress management improve the course of inflammatory bowel disease and disease specific quality of life in distressed patients with ulcerative colitis or Crohn's disease? A randomised controlled trial. Inflammatory Bowel diseases 2011;17(9):1863‐73. - PubMed
Colombel 2010 {published data only}
-
- Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. New England Journal of Medicine 2010;362(15):1383‐95. - PubMed
Cosnes 2013 {published data only}
-
- Cosnes J, Bourrier A, Laharie D, Nahon S, Bouhnik Y, Carbonnel F, et al. Early administration of azathioprine vs conventional management of Crohn's disease: a randomised controlled trial. Gastroenterology 2013;145(4):758‐65. - PubMed
Dewint 2014 {published data only}
-
- Dewint P, Hansen BE, Verhey E, Oldenburg B, Hommes DW, Pierik M, et al. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn's disease: a randomised, double‐blind, placebo controlled trial (ADAFI). Gut 2014;63(2):292‐9. - PubMed
Feagan 2003 {published data only}
-
- Feagan BG, Yan S, Bala M, Bao W, Lichtenstein GR. The effects of infliximab maintenance therapy on health‐related quality of life. American Journal of Gastroenterology 2003;98(10):2232‐8. - PubMed
Ford 2016 {published data only}
Hetzel 2013b {published data only}
-
- Hetzel D, Strauss W, Bernard K. IV iron treatment of iron deficiency anaemia with ferumoxytol in patients with gastrointestinal disorders unable to take oral iron: a randomised controlled trial versus iron sucrose. Journal of Crohn's and Colitis 2013;7:S204.
Leiper 2001 {published data only}
Lichtenstein 2002 {published data only}
-
- Lichtenstein GR, Bala M, Chenglong H, DeWoody K, Schaible T. Infliximab improves quality of life in patients with Crohn’s disease. Inflammatory Bowel Diseases 2002;8(4):237‐43. - PubMed
Loftus 2009 {published data only}
-
- Loftus E, Colombel J, Pollack P, Majethia S, Chen N. Adalimumab treatment associated with rapid and significant improvement in health‐related quality of life in patients with Crohn’s disease. Journal of the Canadian Association of Gastroenterology 2009;23(1).
Loftus 2017 {published data only}
-
- Loftus EV, Colombel JF, Feagan BG, Vermeire S, Sandborn WJ, Sands BE, et al. Long‐term efficacy of vedolizumab for ulcerative colitis. Journal of Crohn's and Colitis 2017;11(4):400‐11. - PubMed
Maragkoudaki 2016 {published data only}
-
- Maragkoudaki M, Chouliaras G, Papadopoulou A, Christaki M. Feasibility and effectiveness of a psycho‐educational program in adolescents with Inflammatory Bowel Disease (IBD). Journal of Pediatric Gastroenterology and Nutrition 2016;61:363.
Mikocka‐Walus 2017 {published data only}
-
- Mikocka‐Walus A, Hughes PA, Bampton P, Gordon A, Campaniello MA, Mavrangelos C, et al. Fluoxetine for maintenance of remission and to improve quality of life in patients with Crohn’s disease: a pilot randomized placebo‐controlled trial. Journal of Crohn's and Colitis 2017;11(4):509‐14. - PMC - PubMed
Minderhoud 2007 {published data only}
NCT01991314 {published data only}
-
- NCT01991314. Treatment of iron deficiency anaemia in adults and adolescents with inflammatory bowel disease using ferrous sulphate: tolerance and effects on haemoglobin, mood, quality of life and fatigue. clinicaltrials.gov/ct2/show/NCT01991314 (first received 25 November 2013).
NCT02148718 {published data only}
-
- NCT02148718. Rapidity of onset of response to adalimumab in luminal Crohn's disease (RAPIDA Study). clinicaltrials.gov/ct2/show/NCT02148718 (first received 28 May 2014).
NCT02162862 {published data only}
-
- NCT02162862. Treating disrupted sleep in individuals with inflammatory bowel disease: a novel adjunctive therapy for chronic inflammatory illness. clinicaltrials.gov/ct2/show/NCT02162862 (first received 13 June 2014).
Paramsothy 2017 {published data only}
-
- Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo‐controlled trial. Lancet 2017;389(10075):1218‐28. - PubMed
Pena Rossi 2009 {published data only}
Persoons 2007 {published data only}
-
- Persoons P, Demyttenaere K, Vandenberghe J, Oudenhove L, Rugeerts P. The evolution of fatigue in patients with Crohn's disease after treatment with infliximab: a prospective study. Gastroenterology 2004;126(4):A476.
Reusch 2016 {published data only}
-
- Reusch A, Weiland R, Gerlich C, Dreger K, Derra C, Mainos D, et al. Self‐management education for rehabilitation inpatients suffering from inflammatory bowel disease: a cluster randomized controlled trial. Health Education Research 2016;31(6):782‐91. - PubMed
Sands 2008 {published data only}
-
- Sands BE, Sandborn WJ, Feagan B, Löfberg R, Hibi T, Wang T, et al. A randomised, double‐blind, sham‐controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastreoenterology 2008;135(2):400‐9. - PubMed
Sands 2013 {published data only}
-
- Sands BE, Katz S, Wolf DC, Feagan BG, Wang T, Gustofson L‐M, et al. A randomised, double‐blind, sham‐controlled study of granulocyte apheresis for moderate to severe Crohn's disease. Gut 2013;62(9):1288‐94. - PubMed
Schmidt 2016 {published data only}
Scholten 2018 {published data only}
-
- Scholten AM, Vermeulen E, Dhonukshe‐Rutten RA, Verhagen T, Visscher A, Olivier A, et al. Surplus vitamin B12 use does not reduce fatigue in patients with irritable bowel syndrome or inflammatory bowel disease: a randomized double‐blind placebo‐controlled trial. Clinical Nutrition European Society for Clinical Nutrition and Metabolism 2018;23:48‐53. - PubMed
Schreiber 2007 {published data only}
-
- Schreiber S, Keshavarzian A, Isaacs KL, Schollenberger J, Guzman JP, Orlandi C, et al. A randomised, placebo‐controlled, phase II study of tetomilast in active ulcerative colitis. Gastroenterology 2007;132(1):76‐86. - PubMed
Smith 2011 {published data only}
Smith 2013 {published data only}
Steinhart 2002 {published data only}
-
- Steinhart AH, Feagan BG, Wong CJ, Vandervoort M, Mikolainis S, Croitoru K, et al. Combined budesonide and antibiotic therapy for active Crohn's disease: a randomised controlled trial. Gastroenterology 2002;123(1):33‐40. - PubMed
Szigethy 2016 {published data only}
-
- Szigethy E, McAuliff K, Strassburger M, Hashash J, Vachon A, Rode NM, et al. Brief behavioral therapy and bupropion for sleep and fatigue in adolescents and young adults with inflammatory bowel disease. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(10):S210.
Targan 2007 {published data only}
-
- Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, et al. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE trial. Gastroenterology 2007;132(5):1672‐83. - PubMed
Valentine 2009 {published data only}
-
- Valentine JF, Fedorak RN, Feagan B, Fredlund P, Schmitt R, Ni P, et al. Steriod‐sparing properties of sargramostim in patients with corticosteroid‐dependent Crohn's disease: a randomised, double‐blind, placebo‐controlled, phase 2 study. Gut 2009;58(10):1354‐62. - PubMed
Van Assche 2012 {published data only}
-
- Assche G, Vermeire S, Ballet V, Gabriels F, Noman M, D'Haens G, et al. Switch to adalimumab in patients with Crohn's disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012;61(2):229‐34. - PubMed
Vermeire 2017 {published data only}
-
- Vermeire S, Loftus EV, Colombel JF, Feagan BG, Sandborn WJ, Sands BE. Long‐term efficacy of vedolizumab for Crohn’s Disease. Journal of Crohn's and Colitis 2017;11(4):412‐24. - PubMed
References to studies awaiting assessment
Ghosh 2019 {published data only}
-
- Ghosh S, Aberra F, Cross R, Zhou W, Chen N, Lee WJ, et al. Effect of upadacitinib on patient‐reported symptoms by the new ulcerative colitis symptoms questionnaire (UC‐SQ) in patients with moderate to severe ulcerative colitis: data from the Phase 2b study U‐ACHIEVE. Journal of Crohn's and Colitis 2019;13:S247‐S248.
-
- NCT02819635. A multicenter, randomized, double‐blind, placebo‐controlled study to evaluate the safety and efficacy of upadacitinib (ABT‐494) for induction and maintenance therapy in subjects with moderately to severely active ulcerative colitis. clinicaltrials.gov/ct2/show/NCT02819635 (first received 30 June 2016).
Louis 2019 {published data only}
-
- Louis E, Sandborn WJ, D'Haens G, Baert F, Kalabic J, Wallace K, et al. Effect of risankizumab on improved and sustained quality of life in patients with moderate to severe Crohn's disease: phase 2 trial results. Journal of Crohn's and Colitis 2019;13:S291‐S292.
-
- NCT02031276. A phase II, multicenter, randomized, double‐blind, multiple dose, placebo‐controlled, parallel‐group study to evaluate the efficacy, pharmacokinetics, and safety of BI 655066/ABBV‐066 (risankizumab), and IL‐23 p19 antagonist monoclonal antibody in patients with ,moderately to severely active Crohn's disease who are naïve to, or were previously treated with, anti‐TNF therapy. clinicaltrials.gov/ct2/show/NCT02031276 (first received 9 January 2014).
O' Connor 2019 {published data only}
-
- NCT02709434. The efficacy of a structured psychoeducational inflammatory bowel disease group on the control of fatigue in an adult outpatient setting for patients with objectively quiescent disease. ClinicalTrials.gov/show/NCT02709434 (first received 16 March 2016).
-
- O'Connor A, Ratnakumaran R, Warren L, Pullen D, Errington A, Gracie DJ, et al. Randomized controlled trial: a pilot study of a psychoeducational intervention for fatigue in patients with quiescent inflammatory bowel disease. Therapeutic Advances in Chronic Disease 2019;10:1‐12. [DOI: 10.1177/2040622319838439] - DOI - PMC - PubMed
Sands 2018 {published data only}
-
- Sands BE, Pires A, Gasink C, Laliman V, Han C, Feagan BG. Post hoc analysis of the impact of ustekinumab treatment on specific items of the Inflammatory Bowel Disease Questionnaire in the Uniti‐1 & 2 programs. Gastroenterology 2018;154:S‐811.
Tew 2019 {published data only}
-
- ISRCTN13021107. A randomised controlled trial investigating the feasibility and acceptability of high‐intensity interval training and moderate‐intensity continuous training in adults with inactive or mildly active Crohn’s disease. www.isrctn.com/ISRCTN13021107 (first received 2 December 2015).
References to ongoing studies
ACTN12617000586314P {published data only}
-
- ACTRN12617000586314P. A prospective randomized multicentre study to evaluate the effect of intravenous iron infusion compared to oral iron supplementation on the quality of life in inflammatory bowel disease with non‐anaemia hypoferritinanaemia. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372758 (first received 17 April 2017).
ACTRN12619000150145 {published data only}
-
- ACTRN12619000150145. Influence of extra virgin olive oil intake on disease activity and gut microbiota profile of community dwelling adults with ulcerative colitis in comparison to healthy subjects. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375208 (first received 24 January 2019).
EudraCT Number: 2008‐004277‐17 {published data only}
-
- EudraCT Number: 2008‐004277‐17. The effectiveness and tolerability of GlobiFer (haem iron) tablets compared to ferrous sulphate tablets in inflammatory bowel disease: a randomised‐controlled trial. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐004277‐17/GB (first received 15 March 2010).
EudraCT Number: 2011‐002122‐43 {published data only}
-
- EudraCT Number: 2011‐002122‐43. Iron therapy in IBD patients with normal levels of haemoglobin and chronic fatigue. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=2011‐00212... (first received).
EudraCT Number: 2012‐005644‐26 {published data only}
-
- EUCTR2012‐005644‐26‐BE. Administration of intravenous ferric carboxymaltose to children with IBD. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=EUCTR2012‐... (first received ).
ISRCTN11470370 {published data only}
-
- ISRCTN11470370. Effects of a 6‐month practical resistance training programme on muscle function and bone mineral density in adults with inactive or mildly active Crohn’s disease: Study protocol for a randomised controlled trial. http://www.isrctn.com/ISRCTN11470370 (first received 11 December 2017).
NCT02193750 {published data only}
-
- NCT02193750. Assessing the tolerability of oligosaccharide supplementation in patients with Crohn's disease: A randomized, controlled trial. https://clinicaltrials.gov/ct2/show/NCT02193750 (first received 18 July 2014).
NCT02208310 {published data only}
-
- NCT02208310. A randomised controlled trial of high‐dose vitamin D in Crohn's disease. https://clinicaltrials.gov/ct2/show/NCT02208310 (first received 5 August 2014).
NCT02517151 {published data only}
-
- NCT02517151. Effects of iron therapy in patients with chronic fatigue and IBD. https://clinicaltrials.gov/ct2/show/NCT02517151 (first received 6 August 2015).
NCT02704624 {published data only}
-
- NCT02704624. The impact of serum vitamin D and calcium levels on the body composition, bone mineral density, muscle strength, exercise tolerance, fatigue and inflammatory activity in patients with Crohn's disease: a randomized controlled trial. https://ClinicalTrials.gov/show/NCT02704624 (first received 10 March 2016).
NCT02707068 {published data only}
-
- NCT02707068. Quality Of LIfe Tool for IBD (QOLITI): pilot testing of a self‐administered intervention to target psychological distress in inflammatory bowel disease. https://clinicaltrials.gov/show/NCT02707068 (first received 14 March 2016).
NCT02772965 {published data only}
-
- NCT02772965. A randomized, double‐blind, placebo‐controlled, multi‐center pragmatic clinical trial to evaluate the effectiveness of low dose oral methotrexate in patients with pediatric Crohn's disease initiating anti‐TNF therapy. https://clinicaltrials.gov/ct2/show/NCT02772965 (first received 16 May 2016).
NCT02849717 {published data only}
-
- NCT02849717. Pre‐habilitation exercise intervention for patients scheduled for colorectal surgical resection. clinicaltrials.gov/ct2/show/NCT02849717 (first received 29 July 2016).
NCT02861053 {published data only}
-
- NCT02861053. Inflammatory Bowel Disease: Could a regular physical activity reduce patients fatigue?. https://clinicaltrials.gov/ct2/show/NCT02861053 (first received 10 August 2016).
NCT02891226 {published data only}
-
- NCT02891226. A phase 2, multicenter, randomized, parallel‐arm, placebo‐controlled study of LY3074828 in subjects with active Crohn's Disease (SERENITY). https://clinicaltrials.gov/ct2/show/NCT02891226 (first received 7 September 2016).
NCT02963246 {published data only}
-
- NCT02963246. Effects of a mindfulness therapy intervention for individuals with inflammatory bowel disease: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT02963246 (first received 15 November 2016).
NCT03104413 {published data only}
-
- NCT03104413. A multicenter, randomized, double‐blind, placebo‐controlled induction study to assess the efficacy and safety of Risankizumab in subjects with moderately to severely active Crohn's disease who failed prior biologic treatment. https://clinicaltrials.gov/show/NCT03104413 (first received 7 April 2017).
NCT03105102 {published data only}
-
- NCT03105102. A multicenter, randomized, double‐blind, placebo controlled 52‐week maintenance and an open‐label extension study of the efficacy and safety of Risankizumab in subjects with Crohn's disease who responded to induction treatment in M16‐006 or M15‐991; or completed M15‐989. ClinicalTrials.gov/show/NCT03105102 (first received 7 April 2017).
NCT03105128 {published data only}
-
- NCT03105128. A multicenter, randomized, double‐blind, placebo controlled induction study of the efficacy and safety of Risankizumab in subjects with moderately to severely active Crohn's disease. ClinicalTrials.gov/show/NCT03105128 (first received 7 April 2017).
NCT03107793 {published data only}
-
- NCT03107793. Study of treat to target versus routine care maintenance strategies in Crohn's disease patients treated with Ustekinumab. clinicaltrials.gov/ct2/show/NCT03107793 (first received 11 April 2017).
NCT03162575 {published data only}
-
- NCT03162575. The possible beneficial effects of mindfulness‐based cognitive therapy (MBCT) in fatigued adult patients with Inflammatory Bowel Disease (IBD). clinicaltrials.gov/ct2/show/NCT03162575 (first received 22 May 2017).
NCT03266484 {published data only}
-
- NCT03266484. Effect of dietary therapy with a probiotic mixture on the gut microbiome and fatigue symptoms in patients with quiescent inflammatory bowel disease ‐ A clinical trial. clinicaltrials.gov/ct2/show/NCT03266484 (first received 30 August 2017).
NCT03345823 {published data only}
-
- NCT03345823. A multicenter, randomized, double‐blind, placebo‐controlled maintenance and long‐term extension study of the efficacy and safety of Upadacitinib (ABT‐494) in subjects with Crohn's disease who completed the studies M14‐431 or M14‐433. clinicaltrials.gov/ct2/show/NCT03345823 (first received 17 November 2017).
NCT03345836 {published data only}
-
- NCT03345836. A multicenter, randomized, double‐blind, placebo‐controlled induction study of the efficacy and safety of Upadacitinib (ABT‐494) in subjects with moderately to severely active Crohn's disease who have inadequately responded to or are intolerant to biologic therapy. clinicaltrials.gov/ct2/show/NCT03345836 (first received 17 November 2017).
NCT03345849 {published data only}
-
- NCT03345849. A multicenter, randomized, double‐blind, placebo‐controlled induction study of the efficacy and safety of Upadacitinib (ABT‐494) in subjects with moderately to severely active Crohn's disease who have inadequately responded to or are Intolerant to conventional and/or biologic therapies. clinicaltrials.gov/ct2/show/NCT03345849 (first received 17 November 2017).
NCT03398135 {published data only}
-
- NCT03398135. A multicenter, randomized, double‐blind, placebo controlled 52‐week maintenance and an open‐label extension study of the efficacy and safety of Risankizumab in subjects with ulcerative colitis who responded to induction treatment in M16‐067 or M16‐065. clinicaltrials.gov/ct2/show/NCT03398135 (first received 12 January 2018).
NCT03398148 {published data only}
-
- NCT03398148. A multicenter, randomized, double‐blind, placebo controlled induction study to evaluate the efficacy and safety of Risankizumab in subjects with moderately to severely active ulcerative colitis who have failed prior biologic therapy. clinicaltrials.gov/ct2/show/NCT03398148 (first received 12 January 2018).
NCT03456752 {published data only}
-
- NCT03456752. The Impact of perioperative dexamethasone on postoperative outcome in inflammatory bowel diseases. clinicaltrials.gov/ct2/show/NCT03456752 (first received 7 March 2018).
NCT03466411 {published data only}
-
- NCT03466411. A phase 2/3, randomized, double‐blind, placebo‐ and active‐controlled, parallel‐group, multicenter protocol to evaluate the efficacy and safety of Guselkumab in participants with moderately to severely active Crohn's disease. clinicaltrials.gov/ct2/show/NCT03466411 (first received 15 March 2018).
NCT03574948 {published data only}
-
- NCT03574948. Multicentric, double‐blind, placebo controlled clinical trial with 5‐hydroxytryptophan (5‐HTP) in patients with inflammatory bowel disease in clinical and biologic remission: effect on fatigue scores. clinicaltrials.gov/ct2/show/NCT03574948 (first received 2 July 2018).
Additional references
Almeida 2016
Bager 2012
-
- Bager P, Befrits R, Wikman O, Lindgren S, Moum B, Hjortswang H, et al. Fatigue in out‐patients with inflammatory bowel disease is common and multifactorial. Alimentary Pharmacology and Therapeutics 2012;35(1):133‐41. - PubMed
Bennett 2009
Bennett 2016
Bernstein 2010
-
- Bernstein C, Fried M, Krabshuis JH, Cohen H, Eliakim R, Fedail S, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflammatory Bowel Diseases 2010;16(1):112‐24. - PubMed
Casati 2000
-
- Casati J, Toner BB, Rooy EC, Drossman DA, Maunder RG. Concerns of patients with inflammatory bowel disease: a review of emerging themes. Digestive Diseases and Sciences 2000;45(1):26‐31. - PubMed
Casellas 2001
-
- Casellas F, López‐Vivancos J, Badia X, Vilaseca J, Malagelada JR. Influence of inflammatory bowel disease on different dimensions of quality of life. European Journal of Gastroenterology and Hepatology 2001;13(5):567‐72. - PubMed
Cella 2002
-
- Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution‐based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. Journal of Pain and Symptom Management 2002;24(6):547‐61. - PubMed
Cella 2005
-
- Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. Journal of Rheumatology 2005;32(5):811‐9. - PubMed
Chalder 1993
-
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. Journal of Psychosomatic Research 1993;37(2):147‐53. - PubMed
Coteur 2009
-
- Coteur G, Feagan B, Keininger DL, Kosinski M. Evaluation of the meaningfulness of health‐related quality of life improvements as assessed by the SF‐36 and the EQ‐5D VAS in patients with active Crohn’s disease. Alimentary Pharmacology & Therapeutics 2009;29(9):1032‐41. - PubMed
Cramp 2012
Cramp 2013
Cronin 2005
-
- Cronin CC, Shanahan F. Understanding symptoms and signs in inflammatory bowel disease. In: Targan SR, Shanahan F, Karp LC editor(s). Inflammatory Bowel Disease: From Bench to Bedside. Springer, 2005:253‐67.
Czuber‐Dochan 2014a
-
- Czuber‐Dochan W, Norton C, Bredin F, Darvell M, Nathan I, Terry H. Healthcare professionals' perceptions of fatigue experienced by people with IBD. Journal of Crohn's and Colitis 2014;8(8):835‐44. - PubMed
Czuber‐Dochan 2014b
-
- Czuber‐Dochan W, Norton C, Bredin F, Forbes A, Nathan I, Berliner S, et al. Assessing fatigue in patients with inflammatory bowel disease. Gastrointestinal Nursing 2014;12(8):13‐21.
Czuber‐Dochan 2014c
-
- Czuber‐Dochan W, Norton C, Bassett P, Berliner S, Bredin F, Darvell M, et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD‐F) patient self‐assessment scale. Journal of Crohn's and Colitis 2014;8(11):1398‐406. - PubMed
Czuber‐Dochan 2013a
-
- Czuber‐Dochan W, Ream E, Norton C. Review article: description and management of fatigue in inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2013;37(5):505‐16. - PubMed
Czuber‐Dochan 2013b
-
- Czuber‐Dochan W, Dibley LB, Terry H, Ream E, Norton C. The experience of fatigue in people with inflammatory bowel disease: an exploratory study. Journal of Advanced Nursing 2013;69(9):1987‐99. - PubMed
Day 2016
De Rooy 2001
-
- Rooy EC, Toner BB, Maunder RG, Greenberg GR, Baron D, Steinhart AH, et al. Concerns of patients with inflammatory bowel disease: results from a clinical population. American Journal of Gastroenterology 2001;96(6):1816‐21. - PubMed
Deek 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane–handbook.org..
Dittner 2004
-
- Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. Journal of Psychosomatic Research 2004;56(2):157‐70. - PubMed
Drossman 1989
-
- Drossman DA, Patrick DL, Mitchell CM, Zagami EA, Appelbaum MI. Health‐related quality of life in inflammatory bowel disease. Digestive Diseases and Sciences 1989;34(9):1379‐86. - PubMed
Elbers 2015
Farrell 2013
-
- Farrell D. Symptom Burden in Individuals with Inflammatory Bowel Disease: a Mixed Methods Study [PhD thesis]. University College Cork, Ireland, 2013.
Farrell 2016
Fisk 1994
-
- Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clinical Infectious Diseases 1994;18(Supplement 1):S79‐83. - PubMed
Fisk 2002
-
- Fisk JD, Doble SE. Construction and validation of a fatigue impact scale for daily administration (D‐FIS). Quality of Life Research 2002;11(3):263‐72. - PubMed
Garcia‐Vega 2004
-
- Garcia‐Vega E, Fernandez‐Rodriguez C. A stress management programme for Crohn’s disease. Behaviour Research and Therapy 2004;42(4):367‐83. - PubMed
Gibbons 2018
Goedendorp 2009
Goligher 2008
-
- Goligher EC, Pouchot J, Brant R, Kherani RB, Avina‐Zubieta JA, Lacaille D, et al. Minimal clinically important difference for 7 measures of fatigue in patients with systemic lupus erythematosus. Journal of Rheumatology 2008;35(4):635‐42. - PubMed
Graff 2011
-
- Graff LA, Vincent N, Walker JR, Clara I, Carr R, Ediger J, et al. A population‐based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflammatory Bowel Diseases 2011;17(9):1882‐9. - PubMed
Heine 2015
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hjollund 2007
Irvine 1999
-
- Irvine EJ. Development and subsequent refinement of the inflammatory bowel disease questionnaire: a quality‐of‐life instrument for adult patients with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition 1999;28(4):S23‐7. - PubMed
Jelsness‐Jørgensen 2011b
Jelsness‐Jørgensen 2012
Jelsness‐Jørgensen 2011a
-
- Jelsness‐Jørgensen L‐P, Bernklev T, Henriksen M, Torp R, Moum BA. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflammatory Bowel Diseases 2011;17(7):1564‐72. - PubMed
Jelsness‐Jørgensen 2011c
-
- Jelsness‐Jørgensen L‐P, Bernklev T, Henriksen M, Torp R, Moum BA. Chronic fatigue is associated with impaired health‐related quality of life in inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2011;33(1):106‐14. - PubMed
Krupp 1989
-
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989;46(10):1121‐3. - PubMed
Lai 2003
-
- Lai J‐S, Cella D, Chang C‐H, Bode RK, Heinemann AW. Item banking to improve, shorten and computerize self‐reported fatigue: an illustration of steps to create a core item bank from the FACIT‐Fatigue Scale. Quality of Life Research 2003;12(5):485‐501. - PubMed
Larun 2017
Lee 1991
-
- Lee KA, Hicks G, Nino‐Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Research 1991;36(3):291‐8. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Minderhoud 2003
-
- Minderhoud IM, Oldenburg B, Dam PS, Berge Henegouwen GP. High prevalence of fatigue in quiescent inflammatory bowel disease is not related to adrenocortical insufficiency. American Journal of Gastroenterology 2003;98(5):1088‐93. - PubMed
Minton 2010
Molodecky 2012
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46‐54.e42. - PubMed
Mücke 2015
Opheim 2014
-
- Opheim R, Fagermoen MS, Bernklev T, Jelsness‐Jorgensen L‐P, Moum B. Fatigue interference with daily living among patients with inflammatory bowel disease. Quality of Life Research 2014;23(2):707‐17. - PubMed
Piper 1998
-
- Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncology Nursing Forum 1998;25(4):677‐84. - PubMed
Pouchot 2008
Preston 2012
Price 2008
Pucci 2007
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Smets 1996
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stone 2008
-
- Stone P, Minton O. Cancer‐related fatigue. European Journal of Cancer 2008;44(8):1097‐104. - PubMed
Tack 1991
-
- Tack BB. Dimensions and Correlates of Fatigue in Older Adults with Rheumatoid Arthritis [PhD thesis]. San Francisco: University of California, 1991.
Tejani 2012
Van Langenberg 2010
-
- Langenberg DR, Gibson PR. Systematic review: fatigue in inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2010;32(2):131‐43. - PubMed
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. - PubMed
White 2014
Wilson 2012
-
- Wilson B, Lönnfors S, Vermeire S. The true impact of IBD: a European Crohn's and Ulcerative Colitis patient life. IMPACT Survey 2010‐2011. 21st Century Challenges Autoimmune Diseases. European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA), Brussels, Belgium; European Crohn’s and Colitis Organization (ECCO); 2012; Vienna, Austria. 2012.
Yellen 1997
-
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anaemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain and Symptom Management 1997;13(2):63‐74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

