Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: A systematic review and meta-analysis
- PMID: 32297988
- PMCID: PMC7262144
- DOI: 10.1002/jmv.25898
Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: A systematic review and meta-analysis
Abstract
Following the demonstration of the efficacy of hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 in vitro, many trials started to evaluate its efficacy in clinical settings. However, no systematic review and meta-analysis have addressed the issue of the safety and efficacy of hydroxychloroquine (HCQ) in coronavirus disease 2019. We conducted a systematic review and meta-analysis with the objectives of evaluation of safety and efficacy of HCQ alone or in combination in terms of "time to clinical cure," "virological cure," "death or clinical worsening of disease," "radiological progression," and safety. RevMan was used for meta-analysis. We searched 16 literature databases out of which seven studies (n = 1358) were included in the systematic review. In terms of clinical cure, two studies reported possible benefit in "time to body temperature normalization" and one study reported less "cough days" in the HCQ arm. Treatment with HCQ resulted in less number of cases showing the radiological progression of lung disease (odds ratio [OR], 0.31, 95% confidence interval [CI], 0.11-0.9). No difference was observed in virological cure (OR, 2.37, 95% CI, 0.13-44.53), death or clinical worsening of disease (OR, 1.37, 95% CI, 1.37-21.97), and safety (OR, 2.19, 95% CI, 0.59-8.18), when compared with the control/conventional treatment. Five studies reported either the safety or efficacy of HCQ + azithromycin. Although seems safe and effective, more data are required for a definitive conclusion. HCQ seems to be promising in terms of less number of cases with radiological progression with a comparable safety profile to control/conventional treatment. We need more data to come to a definite conclusion.
Keywords: 2019-nCoV; COVID-19; SARS CoV-2; hydroxychloroquine; meta-analysis.
© 2020 Wiley Periodicals, Inc.
Conflict of interest statement
All the authors declared that there are no conflict of interests.
Figures
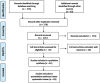
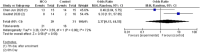
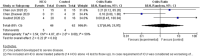


Comment in
-
Conduction abnormalities in hydroxychloroquine add on therapy to lopinavir/ritonavir in COVID-19.J Med Virol. 2020 Nov;92(11):2322-2324. doi: 10.1002/jmv.26004. Epub 2020 May 22. J Med Virol. 2020. PMID: 32401368 Free PMC article. No abstract available.
-
Clinical and biological data on the use of hydroxychloroquine against SARS-CoV-2 could support the role of the NLRP3 inflammasome in the pathogenesis of respiratory disease.J Med Virol. 2021 Jan;93(1):124-126. doi: 10.1002/jmv.26217. Epub 2020 Jul 6. J Med Virol. 2021. PMID: 32579244 Free PMC article. No abstract available.
References
-
- Coronavirus Disease (COVID‐19) ‐ events as they happen. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/events‐a.... Accessed March 31, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

