Bridge-Enhanced Anterior Cruciate Ligament Repair Is Not Inferior to Autograft Anterior Cruciate Ligament Reconstruction at 2 Years: Results of a Prospective Randomized Clinical Trial
- PMID: 32298131
- PMCID: PMC7227128
- DOI: 10.1177/0363546520913532
Bridge-Enhanced Anterior Cruciate Ligament Repair Is Not Inferior to Autograft Anterior Cruciate Ligament Reconstruction at 2 Years: Results of a Prospective Randomized Clinical Trial
Abstract
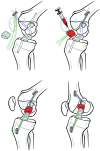
Background: Preclinical studies suggest that for complete midsubstance anterior cruciate ligament (ACL) injuries, a suture repair of the ACL augmented with a protein implant placed in the gap between the torn ends (bridge-enhanced ACL repair [BEAR]) may be a viable alternative to ACL reconstruction (ACLR).
Hypothesis: We hypothesized that patients treated with BEAR would have a noninferior patient-reported outcomes (International Knee Documentation Committee [IKDC] Subjective Score; prespecified noninferiority margin, -11.5 points) and instrumented anteroposterior (AP) knee laxity (prespecified noninferiority margin, +2-mm side-to-side difference) and superior muscle strength at 2 years after surgery when compared with patients who underwent ACLR with autograft.
Study design: Randomized controlled trial; Level of evidence, 1.
Methods: One hundred patients (median age, 17 years; median preoperative Marx activity score, 16) with complete midsubstance ACL injuries were enrolled and underwent surgery within 45 days of injury. Patients were randomly assigned to receive either BEAR (n = 65) or autograft ACLR (n = 35 [33 with quadrupled semitendinosus-gracilis and 2 with bone-patellar tendon-bone]). Outcomes-including the IKDC Subjective Score, the side-to-side difference in instrumented AP knee laxity, and muscle strength-were assessed at 2 years by an independent examiner blinded to the procedure. Patients were unblinded after their 2-year visit.
Results: In total, 96% of the patients returned for 2-year follow-up. Noninferiority criteria were met for both the IKDC Subjective Score (BEAR, 88.9 points; ACLR, 84.8 points; mean difference, 4.1 points [95% CI, -1.5 to 9.7]) and the side-to-side difference in AP knee laxity (BEAR, 1.61 mm; ACLR, 1.77 mm; mean difference, -0.15 mm [95% CI, -1.48 to 1.17]). The BEAR group had a significantly higher mean hamstring muscle strength index than the ACLR group at 2 years (98.2% vs 63.2%; P < .001). In addition, 14% of the BEAR group and 6% of the ACLR group had a reinjury that required a second ipsilateral ACL surgical procedure (P = .32). Furthermore, the 8 patients who converted from BEAR to ACLR in the study period and returned for the 2-year postoperative visit had similar primary outcomes to patients who had a single ipsilateral ACL procedure.
Conclusion: BEAR resulted in noninferior patient-reported outcomes and AP knee laxity and superior hamstring muscle strength when compared with autograft ACLR at 2-year follow-up in a young and active cohort. These promising results suggest that longer-term studies of this technique are justified.
Registration: NCT02664545 (ClinicalTrials.gov identifier).
Keywords: ACL reconstruction; ACL repair; BEAR; anterior cruciate ligament; bridge-enhanced ACL repair; human; scaffold-enhanced ACL repair.
Conflict of interest statement
One or more of the authors has declared the following potential conflict of interest or source of funding: This study received funding support from the Translational Research Program at Boston Children’s Hospital, the Children’s Hospital Orthopaedic Surgery Foundation, the Children’s Hospital Sports Medicine Foundation, the Football Players Health Study at Harvard University, and the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases through grants R01-AR065462 and R01-AR056834. M.M.M. is a founder, paid consultant, and equity holder in Miach Orthopaedics, Inc, which was formed to work on upscaling production of the BEAR scaffold. M.M.M. maintained a conflict-of-interest management plan that was approved by Boston Children’s Hospital and Harvard Medical School during the conduct of the trial, with oversight by both conflict-of-interest committees and the institutional review board of Boston Children’s Hospital, as well as the US Food and Drug Administration. B.C.F. is an assistant editor for
Figures
Comment in
-
In Complete ACL Tears, Bridge-Enhanced ACL Repair Was Noninferior to ACL Reconstruction for Symptoms and Functioning and Knee Laxity at 2 Years.J Bone Joint Surg Am. 2021 Feb 17;103(4):358. doi: 10.2106/JBJS.20.02088. J Bone Joint Surg Am. 2021. PMID: 33369980 No abstract available.
References
-
- Abourezk MN, Ithurburn MP, McNally MP, et al. Hamstring strength asymmetry at 3 years after anterior cruciate ligament reconstruction alters knee mechanics during gait and jogging. Am J Sports Med. 2017;45:97-105. - PubMed
-
- Andernord D, Desai N, Bjornsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43:121-127. - PubMed
-
- Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ; International Knee Documentation Committee. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med. 2006;34:128-135. - PubMed
-
- Arneja S, Leith J. Review article: validity of the KT-1000 knee ligament arthrometer. J Orthop Surg (Hong Kong). 2009;17:77-79. - PubMed
-
- Astur DC, Cachoeira CM, da Silva Vieira T, Debieux P, Kaleka CC, Cohen M. Increased incidence of anterior cruciate ligament revision surgery in paediatric verses adult population. Knee Surg Sports Traumatol Arthrosc. 2018;26:1362-1366. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

