Long-Term Follow-Up Results of Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy and Risk-Adapted Maintenance Approach in Newly Diagnosed Multiple Myeloma
- PMID: 32298201
- PMCID: PMC7587409
- DOI: 10.1200/JCO.19.02515
Long-Term Follow-Up Results of Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy and Risk-Adapted Maintenance Approach in Newly Diagnosed Multiple Myeloma
Erratum in
-
Errata.J Clin Oncol. 2020 Aug 10;38(23):2702. doi: 10.1200/JCO.20.01986. J Clin Oncol. 2020. PMID: 32755511 Free PMC article. No abstract available.
Abstract
Purpose: The combination of lenalidomide, bortezomib, and dexamethasone (RVD) is a highly effective and convenient induction regimen for both transplantation-eligible and -ineligible patients with myeloma. Here, we present the largest cohort of patients consecutively treated with RVD induction therapy followed by risk-adapted maintenance therapy with the longest follow-up and important information on long-term outcomes.
Patients and methods: We describe 1,000 consecutive patients with newly diagnosed myeloma treated with RVD induction therapy from January 2007 until August 2016. Demographic and clinical characteristics and outcomes data were obtained from our institutional review board-approved myeloma database. Responses and progression were evaluated per International Myeloma Working Group Uniform Response Criteria.
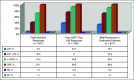
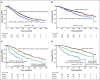
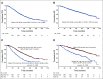
Results: The overall response rate was 97.1% after induction therapy and 98.5% after transplantation, with 89.9% of patients achieving a very good partial response (VGPR) or better and 33.3% achieving stringent complete response after transplantation at a median follow-up time of 67 months. The estimated median progression-free survival time was 65 months (95% CI, 58.7 to 71.3 months) for the entire cohort, 40.3 months (95% CI, 33.5 to 47 months) for high-risk patients, and 76.5 months (95% CI, 66.9 to 86.2 months) for standard-risk patients. The median overall survival (OS) time for the entire cohort was 126.6 months (95% CI, 113.3 to 139.8 months). The median OS for high-risk patients was 78.2 months (95% CI, 62.2 to 94.2 months), whereas it has not been reached for standard-risk patients. Five-year OS rates for high-risk and standard-risk patients were 57% and 81%, respectively, and the 10-year OS rates were 29% and 58%, respectively.
Conclusion: RVD is an induction regimen that delivers high response rates (VGPR or better) in close to 90% of patients after transplantation, and risk-adapted maintenance can deliver unprecedented long-term outcomes. This study includes the largest cohort of patients treated with RVD reported to date with long follow-up and demonstrates the ability of 3-drug induction regimens in patients with newly diagnosed multiple myeloma to result in a substantial survival benefit.
Figures
References
-
- Munjuluri A, Fillmore N, Cirstea D, et al. With equal access, African Americans with non-del17p multiple myeloma have superior overall survival, but del17p still carries poor prognosis across race: A VA study. Blood. 2019;134(suppl 1):4388.
-
- Kaiser MF, Jenner M, Cairns D, et al. Outcomes of transplant-eligible newly diagnosed ultra-high risk myeloma patients treated in the NCRI Myeloma XI Trial indicate the need for early treatment stratification and novel treatment approaches. Blood. 2019;134(suppl 1):604.
-
- Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28:690–693. - PubMed
-
- Zaccaria GM, Capra A, Petrucci MT, et al. Predictive model of early relapse in newly diagnosed multiple myeloma: Analysis from a pooled dataset. Blood. 2019;134(suppl 1):2130.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

