Long-Term Outcomes From a Randomized Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells in Relapsed Chronic Lymphocytic Leukemia
- PMID: 32298202
- PMCID: PMC8265376
- DOI: 10.1200/JCO.19.03237
Long-Term Outcomes From a Randomized Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells in Relapsed Chronic Lymphocytic Leukemia
Abstract
Purpose: To describe long-term outcomes of anti-CD19 chimeric antigen receptor T (CART) cells in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
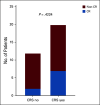
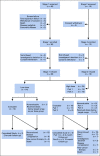
Methods: Between January 2013 and June 2016, 42 patients with relapsed or refractory CLL were enrolled in this study and 38 were infused with anti-CD19 CART cells (CART-19). Of these, 28 patients were initially randomly assigned to receive a low (5 × 107) or high (5 × 108) dose of CART-19, and 24 were evaluable for response assessment. After an interim analysis, 10 additional patients received the selected (high) dose and of these, eight were evaluable for response. Patients were followed for a median 31.5 months (range, 2 to 75 months).
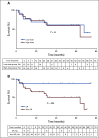
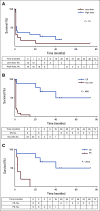
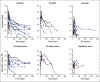
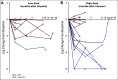
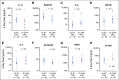
Results: At 4 weeks, the complete and overall responses for the 32 evaluable patients were 28% (90% CI, 16% to 44%) and 44% (90% CI, 29% to 60%), respectively. The median overall survival (OS) for all patients was 64 months; there was no statistically significant difference between low- and high-dose groups (P = .84). Regardless of dose, prolonged survival was observed in patients who achieved a CR versus those who did not (P = .035), with median OS not reached in patients with CR versus 64 months in those without CR. The median progression-free survival was 40.2 months in patients with CR and 1 month in those without a CR (P < .0001). Toxicity was comparable in both dose groups.
Conclusion: In patients with advanced CLL, a 5 × 108 dose of CART-19 may be more effective than 5 × 107 CART-19 at inducing CR without excessive toxicity. Attainment of a CR after CART-19 infusion, regardless of cell dose, is associated with longer OS and progression-free survival in patients with relapsed CLL.
Trial registration: ClinicalTrials.gov NCT02640209.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

