IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury
- PMID: 32298238
- PMCID: PMC7324198
- DOI: 10.1172/JCI136908
IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury
Erratum in
-
IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury.J Clin Invest. 2023 Mar 1;133(5):e169500. doi: 10.1172/JCI169500. J Clin Invest. 2023. PMID: 36856118 Free PMC article. No abstract available.
Abstract
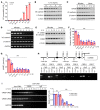
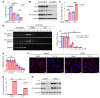
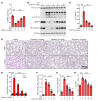
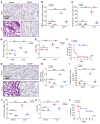
Unchecked inflammation is a hallmark of inflammatory tissue injury in diseases such as acute respiratory distress syndrome (ARDS). Yet the mechanisms of inflammatory lung injury remain largely unknown. Here we showed that bacterial endotoxin lipopolysaccharide (LPS) and cecal ligation and puncture-induced (CLP-induced) polymicrobial sepsis decreased the expression of transcription factor cAMP response element binding (CREB) in lung endothelial cells. We demonstrated that endothelial CREB was crucial for VE-cadherin transcription and the formation of the normal restrictive endothelial adherens junctions. The inflammatory cytokine IL-1β reduced cAMP generation and CREB-mediated transcription of VE-cadherin. Furthermore, endothelial cell-specific deletion of CREB induced lung vascular injury whereas ectopic expression of CREB in the endothelium prevented the injury. We also observed that rolipram, which inhibits type 4 cyclic nucleotide phosphodiesterase-mediated (PDE4-mediated) hydrolysis of cAMP, prevented endotoxemia-induced lung vascular injury since it preserved CREB-mediated VE-cadherin expression. These data demonstrate the fundamental role of the endothelial cAMP-CREB axis in promoting lung vascular integrity and suppressing inflammatory injury. Therefore, strategies aimed at enhancing endothelial CREB-mediated VE-cadherin transcription are potentially useful in preventing sepsis-induced lung vascular injury in ARDS.
Keywords: Inflammation; Innate immunity; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

