Repurposing anti-diabetic drug "Sitagliptin" as a novel virulence attenuating agent in Serratia marcescens
- PMID: 32298346
- PMCID: PMC7162429
- DOI: 10.1371/journal.pone.0231625
Repurposing anti-diabetic drug "Sitagliptin" as a novel virulence attenuating agent in Serratia marcescens
Abstract
Background: Serratia marcescens is an emerging pathogen that causes a variety of health care associated infections. S. marcescens is equipped with an arsenal of virulence factors such as biofilm formation, swimming and swarming motilities, prodigiosin, protease and others which enable it to initiate and cause the infection. These virulence factors are orchestrated under the umbrella of an intercellular communication system named Quorum sensing (QS). QS allows bacterial population to synchronize the expression of virulence genes upon detection of a chemical signaling molecule. Targeting bacterial virulence is a promising approach to attenuate bacteria and enhances the ability of immune system to eradicate the bacterial infection. Drug repurposing is an advantageous strategy that confers new applications for drugs outside the scope of their original medical use. This promising strategy offers the use of safe approved compounds, which potentially lowers the costs and shortens the time than that needed for development of new drugs. Sitagliptin is dipeptidyl peptidase-4 (DPP-4) inhibitor, is used to treat diabetes mellitus type II as it increases the production of insulin and decreasing the production of glucagon by the pancreas. We aimed in this study to repurpose sitagliptin, investigating the anti-virulence activities of sitagliptin on S. marcescens.
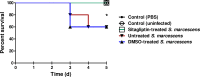
Methods: The effect of sub-inhibitory concentrations of sitagliptin on virulence factors; protease, prodigiosin, biofilm formation, swimming and swarming motilities was estimated phenotypically. The qRT-PCR was used to show the effect of sitagliptin on the expression of QS-regulated virulence genes. The in-vivo protective activity of sitagliptin on S. marcescens pathogenesis was evaluated on mice.
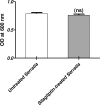
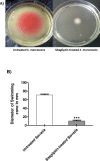
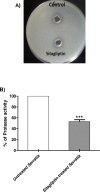
Results: Sitagliptin (1 mg/ml) significantly reduced the biofilm formation, swimming and swarming motilities, prodigiosin and protease. The qRT-PCR confirmed the effect on virulence as shown by down regulating the expression of fimA, fimC, flhC, flhD, bsmB, rssB, rsmA, pigP, and shlA genes. Moreover, the in-vivo findings showed the efficient ability of sitagliptin to weaken S. marcescens pathogenesis.
Conclusion: Sitagliptin is a promising anti-virulence agent against S. marcescens that may be beneficial in the control of healthcare associated infections caused by S. marcescens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Jones RN (2010) Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 51 Suppl 1: S81–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

