PPARα-Dependent Effects of Palmitoylethanolamide Against Retinal Neovascularization and Fibrosis
- PMID: 32298438
- PMCID: PMC7401460
- DOI: 10.1167/iovs.61.4.15
PPARα-Dependent Effects of Palmitoylethanolamide Against Retinal Neovascularization and Fibrosis
Abstract
Purpose: Pathological neovascularization and fibrosis are common pathological changes of many retinal diseases, such as proliferative retinopathy (PR) and age-related macular degeneration (AMD). Treatment modalities for these pathological changes are limited. The purpose of the present study was to test the effects of palmitoylethanolamide (PEA), an endocannabinoid mimetic amide, on retinal neovascularization and fibrosis and to determine its molecular mechanism of action.
Methods: A rat Müller cell line (rMC-1), a mouse model of oxygen-induced retinopathy (OIR), and the very-low-density lipoprotein receptor (VLDLR) knockout mouse model were used. PEA was intraperitoneally injected or orally administrated in animal models. Inflammation and profibrotic changes were evaluated by western blot analysis. Glial fibrillary acidic protein (GFAP) and peroxisome proliferator-activated receptor alpha (PPARα) were measured by RT-PCR and western blot analysis.
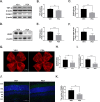
Results: Profibrotic changes were present in OIR and Vldlr-/- retinas. PEA significantly alleviated inflammation and inhibited neovascularization in OIR and Vldlr-/- retinas and suppressed profibrotic changes in OIR and Vldlr-/- retinas. Moreover, PEA potently suppressed Müller gliosis in these retinas. In rMC-1 cells, PEA suppressed Müller gliosis, reduced inflammatory cytokines, and attenuated profibrotic changes. Further, both mRNA and protein levels of PPARα were elevated in the retina under PEA treatment, and the effects of PEA were abolished in Pparα-/- OIR mice.
Conclusions: PEA reduced retinal neovascularization and fibrotic changes and suppressed Müller gliosis in experimental PR and neovascular AMD by activating PPARα. PEA may be a potential treatment for retinopathies with pathological neovascularization and fibrosis.
Conflict of interest statement
Disclosure:
Figures








References
-
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227–1239. - PubMed
-
- Di Zazzo A, Roberti G, Mashaghi A, Abud TB, Pavese D, Bonini S. Use of topical cannabinomimetic palmitoylethanolamide in ocular surface disease associated with antiglaucoma medications. J Ocul Pharmacol Ther. 2017; 33: 670–677. - PubMed
-
- Paterniti I, Di Paola R, Campolo M, et al. .. Palmitoylethanolamide treatment reduces retinal inflammation in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2015; 769: 313–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

