Saliva is a reliable tool to detect SARS-CoV-2
- PMID: 32298676
- PMCID: PMC7194805
- DOI: 10.1016/j.jinf.2020.04.005
Saliva is a reliable tool to detect SARS-CoV-2
Abstract
Objectives: This study analyzed salivary samples of COVID-19 patients and compared the results with their clinical and laboratory data.
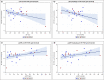
Methods: Salivary samples of 25 COVID-19 patients were analyzed by rRT-PCR. The following data were collected: age, sex, comorbidities, drugs. Lactate dehydrogenase (LDH) and ultrasensitive reactive C protein (usRCP) values were registered on the same day when a salivary swab was collected. Prevalence of positivity in saliva and association between clinical data and the cycle threshold as a semiquantitative indicator of viral load were considered.
Results: Twenty-five subjects were recruited into this study, 17 males and 8 females. The mean age was 61.5 +/- 11.2 years. Cardiovascular and/or dysmetabolic disorders were observed in 65.22% of cases. All the samples tested positive for the presence of SARS-CoV-2, while there was an inverse association between LDH and Ct values. Two patients showed positive salivary results on the same days when their pharyngeal or respiratory swabs showed conversion.
Conclusions: Saliva is a reliable tool to detect SARS-CoV-2. The role of saliva in COVID-19 diagnosis could not be limited to a qualitative detection of the virus, but it may also provide information about the clinical evolution of the disease.
Keywords: COVID-19; Coronavirus; SARS-CoV-2; Saliva; nCoV-2019.
Copyright © 2020. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interests None.
Figures
Comment in
-
Comparison of SARS-CoV-2 detection in nasopharyngeal swab and saliva.J Infect. 2020 Aug;81(2):e145-e147. doi: 10.1016/j.jinf.2020.05.071. Epub 2020 Jun 4. J Infect. 2020. PMID: 32504740 Free PMC article. No abstract available.
-
SARS-CoV-2 RNA detection in tears and conjunctival secretions of COVID-19 patients with conjunctivitis.J Infect. 2020 Sep;81(3):452-482. doi: 10.1016/j.jinf.2020.05.070. Epub 2020 Jun 3. J Infect. 2020. PMID: 32504746 Free PMC article. No abstract available.
-
Rapid Salivary Test suitable for a mass screening program to detect SARS-CoV-2: A diagnostic accuracy study.J Infect. 2020 Sep;81(3):e75-e78. doi: 10.1016/j.jinf.2020.06.042. Epub 2020 Jun 21. J Infect. 2020. PMID: 32579988 Free PMC article. No abstract available.
-
Viral dynamics of SARS-CoV-2 in saliva from infected patients.J Infect. 2020 Sep;81(3):e48-e50. doi: 10.1016/j.jinf.2020.06.059. Epub 2020 Jun 25. J Infect. 2020. PMID: 32593658 Free PMC article. No abstract available.
-
USEFULNESS OF SALIVA SAMPLES FOR DETECTING SARS-CoV-2 RNA AMONG LIVER DISEASE PATIENTS.J Infect. 2021 Jan;82(1):e4-e5. doi: 10.1016/j.jinf.2020.07.017. Epub 2020 Jul 23. J Infect. 2021. PMID: 32711040 Free PMC article. No abstract available.
-
Serial semiquantitative detection of SARS-CoV-2 in saliva samples.J Infect. 2021 Mar;82(3):414-451. doi: 10.1016/j.jinf.2020.10.002. Epub 2020 Oct 6. J Infect. 2021. PMID: 33031835 Free PMC article. No abstract available.
-
Evaluation of the rapid antigen test Panbio COVID-19 in saliva and nasal swabs in a population-based point-of-care study.J Infect. 2021 May;82(5):186-230. doi: 10.1016/j.jinf.2020.12.007. Epub 2020 Dec 9. J Infect. 2021. PMID: 33309541 Free PMC article. No abstract available.
-
Concerns about the clinical usefulness of saliva specimens for the diagnosis of COVID-19.J Infect. 2021 Jul;83(1):119-145. doi: 10.1016/j.jinf.2021.04.007. Epub 2021 Apr 16. J Infect. 2021. PMID: 33872667 Free PMC article. No abstract available.
References
-
- World Health Organization2019-nCoV Situation report – 1 21 January 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- Centers for Disease Control and Prevention (CDC) Update: novel influenza A (H1N1) virus infections – worldwide,Mmay 6, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:453–458. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

