COVID-19: Abnormal liver function tests
- PMID: 32298767
- PMCID: PMC7194951
- DOI: 10.1016/j.jhep.2020.04.006
COVID-19: Abnormal liver function tests
Abstract
Background & aims: Recent data on the coronavirus disease 2019 (COVID-19) outbreak caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has begun to shine light on the impact of the disease on the liver. But no studies to date have systematically described liver test abnormalities in patients with COVID-19. We evaluated the clinical characteristics of COVID-19 in patients with abnormal liver test results.
Methods: Clinical records and laboratory results were obtained from 417 patients with laboratory-confirmed COVID-19 who were admitted to the only referral hospital in Shenzhen, China from January 11 to February 21, 2020 and followed up to March 7, 2020. Information on clinical features of patients with abnormal liver tests were collected for analysis.
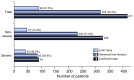
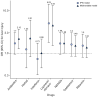
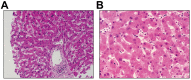
Results: Of 417 patients with COVID-19, 318 (76.3%) had abnormal liver test results and 90 (21.5%) had liver injury during hospitalization. The presence of abnormal liver tests became more pronounced during hospitalization within 2 weeks, with 49 (23.4%), 31 (14.8%), 24 (11.5%) and 51 (24.4%) patients having alanine aminotransferase, aspartate aminotransferase, total bilirubin and gamma-glutamyl transferase levels elevated to more than 3× the upper limit of normal, respectively. Patients with abnormal liver tests of hepatocellular type or mixed type at admission had higher odds of progressing to severe disease (odds ratios [ORs] 2.73; 95% CI 1.19-6.3, and 4.44, 95% CI 1.93-10.23, respectively). The use of lopinavir/ritonavir was also found to lead to increased odds of liver injury (OR from 4.44 to 5.03, both p <0.01).
Conclusion: Patients with abnormal liver tests were at higher risk of progressing to severe disease. The detrimental effects on liver injury mainly related to certain medications used during hospitalization, which should be monitored and evaluated frequently.
Lay summary: Data on liver tests in patients with COVID-19 are scarce. We observed a high prevalence of liver test abnormalities and liver injury in 417 patients with COVID-19 admitted to our referral center, and the prevalence increased substantially during hospitalization. The presence of abnormal liver tests and liver injury were associated with the progression to severe pneumonia. The detrimental effects on liver injury were related to certain medications used during hospitalization, which warrants frequent monitoring and evaluation for these patients.
Keywords: 2019-nCoV; Bilirubin; Critical care; Liver injury; Liver tests; Pneumonia; SARS-Cov-2.
Copyright © 2020 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Comment in
-
Clinical characteristics of COVID-19 patients with abnormal liver tests.J Hepatol. 2020 Sep;73(3):712-713. doi: 10.1016/j.jhep.2020.04.028. Epub 2020 Apr 26. J Hepatol. 2020. PMID: 32348791 Free PMC article. No abstract available.
-
Liver tests abnormalities in COVID-19: trick or treat?J Hepatol. 2020 Nov;73(5):1275-1276. doi: 10.1016/j.jhep.2020.05.033. Epub 2020 May 27. J Hepatol. 2020. PMID: 32473194 Free PMC article. No abstract available.
-
Involvement of liver in COVID-19: systematic review and meta-analysis.Gut. 2021 Apr;70(4):807-809. doi: 10.1136/gutjnl-2020-322072. Epub 2020 Jul 15. Gut. 2021. PMID: 32669289 Free PMC article. No abstract available.
References
-
- Dong Y., Liang X., Yu X. Prognostic value of the dynamic changes in extra vascular lung water index and angiopoietin-2 in severe multiple trauma patients with acute respiratory distress syndromeZhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31:571–576. - PubMed
-
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. Published online February 24, 2020. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

