Performance of immune-based and microbiological tests in children with tuberculosis meningitis in Europe: a multicentre Paediatric Tuberculosis Network European Trials Group (ptbnet) study
- PMID: 32299859
- PMCID: PMC7330130
- DOI: 10.1183/13993003.02004-2019
Performance of immune-based and microbiological tests in children with tuberculosis meningitis in Europe: a multicentre Paediatric Tuberculosis Network European Trials Group (ptbnet) study
Abstract
Introduction: Tuberculous meningitis (TBM) is often diagnostically challenging. Only limited data exist on the performance of interferon-γ release assays (IGRA) and molecular assays in children with TBM in routine clinical practice, particularly in the European setting.
Methods: Multicentre, retrospective study involving 27 healthcare institutions providing care for children with tuberculosis (TB) in nine European countries.
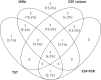
Results: Of 118 children included, 54 (45.8%) had definite, 38 (32.2%) probable and 26 (22.0%) possible TBM; 39 (33.1%) had TBM grade 1, 68 (57.6%) grade 2 and 11 (9.3%) grade 3. Of 108 patients who underwent cranial imaging 90 (83.3%) had at least one abnormal finding consistent with TBM. At the 5-mm cut-off the tuberculin skin test had a sensitivity of 61.9% (95% CI 51.2-71.6%) and at the 10-mm cut-off 50.0% (95% CI 40.0-60.0%). The test sensitivities of QuantiFERON-TB and T-SPOT.TB assays were 71.7% (95% CI 58.4-82.1%) and 82.5% (95% CI 58.2-94.6%), respectively (p=0.53). Indeterminate results were common, occurring in 17.0% of QuantiFERON-TB assays performed. Cerebrospinal fluid (CSF) cultures were positive in 50.0% (95% CI 40.1-59.9%) of cases, and CSF PCR in 34.8% (95% CI 22.9-43.7%). In the subgroup of children who underwent tuberculin skin test, IGRA, CSF culture and CSF PCR simultaneously, 84.4% had at least one positive test result (95% CI 67.8%-93.6%).
Conclusions: Existing immunological and microbiological TB tests have suboptimal sensitivity in children with TBM, with each test producing false-negative results in a substantial proportion of patients. Combining immune-based tests with CSF culture and CSF PCR results in considerably higher positive diagnostic yields, and should therefore be standard clinical practice in high-resource settings.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: R. Basu Roy was a consultant for FIND, Geneva, a non-profit organization, from 2014 to 2016. Conflict of interest: S. Thee has nothing to disclose. Conflict of interest: D. Blázquez-Gamero has nothing to disclose. Conflict of interest: L. Falcón-Neyra has nothing to disclose. Conflict of interest: O. Neth has nothing to disclose. Conflict of interest: A. Noguera-Julian has nothing to disclose. Conflict of interest: C. Lillo has nothing to disclose. Conflict of interest: L. Galli has nothing to disclose. Conflict of interest: E. Venturini has nothing to disclose. Conflict of interest: D. Buonsenso has nothing to disclose. Conflict of interest: F. Götzinger has nothing to disclose. Conflict of interest: N. Martinez-Alier has nothing to disclose. Conflict of interest: S. Velizarova has nothing to disclose. Conflict of interest: F. Brinkmann has nothing to disclose. Conflict of interest: S.B. Welch has nothing to disclose. Conflict of interest: M. Tsolia has nothing to disclose. Conflict of interest: B. Santiago-Garcia has received diagnostic assays free of charge for other projects from Cepheid, and support for conference attendance from GlaxoSmithKline. Conflict of interest: R. Krüger has nothing to disclose. Conflict of interest: M. Tebruegge has received QuantiFERON assays at reduced pricing or free of charge for other TB diagnostics projects from the manufacturer (Cellestis/Qiagen), and has received support for conference attendance from Cepheid.
Figures


References
-
- World Health Organization Global Tuberculosis Report 2018. World Health Organization, Geneva, 2019.
