A minor population of macrophage-tropic HIV-1 variants is identified in recrudescing viremia following analytic treatment interruption
- PMID: 32300019
- PMCID: PMC7211992
- DOI: 10.1073/pnas.1917034117
A minor population of macrophage-tropic HIV-1 variants is identified in recrudescing viremia following analytic treatment interruption
Abstract
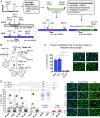
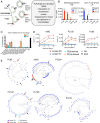
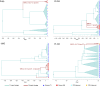
HIV-1 persists in cellular reservoirs that can reignite viremia if antiretroviral therapy (ART) is interrupted. Therefore, insight into the nature of those reservoirs may be revealed from the composition of recrudescing viremia following treatment cessation. A minor population of macrophage-tropic (M-tropic) viruses was identified in a library of recombinant viruses constructed with individual envelope genes that were obtained from plasma of six individuals undergoing analytic treatment interruption (ATI). M-tropic viruses could also be enriched from post-ATI plasma using macrophage-specific (CD14) but not CD4+ T cell-specific (CD3) antibodies, suggesting that M-tropic viruses had a macrophage origin. Molecular clock analysis indicated that the establishment of M-tropic HIV-1 variants predated ATI. Collectively, these data suggest that macrophages are a viral reservoir in HIV-1-infected individuals on effective ART and that M-tropic variants can appear in rebounding viremia when treatment is interrupted. These findings have implications for the design of curative strategies for HIV-1.
Keywords: HIV-1 reservoirs; analytical treatment interruption; macrophages.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Finzi D., et al. , Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997). - PubMed
-
- Koenig S., et al. , Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233, 1089–1093 (1986). - PubMed
-
- Igarashi T., et al. , Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. U.S.A. 98, 658–663 (2001). - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

