Induction of Myogenic Differentiation Improves Chemosensitivity of Chemoresistant Cells in Soft-Tissue Sarcoma Cell Lines
- PMID: 32300280
- PMCID: PMC7136814
- DOI: 10.1155/2020/8647981
Induction of Myogenic Differentiation Improves Chemosensitivity of Chemoresistant Cells in Soft-Tissue Sarcoma Cell Lines
Abstract
Rhabdomyosarcoma (RMS) and rhabdoid tumors (RT) are rare soft-tissue malignancies with the highest incidence in infants, children, and adolescents. Advanced, recurrent, and/or metastatic RMS and RT exhibit poor response to treatment. One of the main mechanisms behind resistance to treatment is believed to be intratumoral heterogeneity. In this study, we investigated the myogenic determination factor 1 (MYOD1) and Noggin (NOG) markers in an embryonal RMS (ERMS) cell line and an RT cell line and the differential response of the MYOD1 and NOG expressing subpopulations to chemotherapy. Importantly, we found that these markers together identify a subpopulation of cells (MYOD1+ NOG+ cells) with primary resistance to Vincristine and Doxorubicin, two commonly used chemotherapies for ERMS and RT. The chemoresistant MYOD1+ NOG+ cells express markers of undifferentiated cells such as myogenin and ID1. Combination of Vincristine with TPA/GSK126, a drug combination shown to induce differentiation of RMS cell lines, is able to partially overcome MYOD1/NOG cells chemoresistance.
Copyright © 2020 Lucy E. Dawson et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

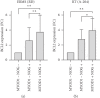
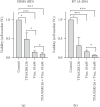
References
-
- Pastore G., Peris-Bonet R., Carli M., Martínez-García C., de Toledo J. S., Steliarova-Foucher E. Childhood soft tissue sarcomas incidence and survival in European children (1978–1997): report from the automated childhood cancer information system project. European Journal of Cancer. 2006;42(13):2136–2149. doi: 10.1016/j.ejca.2006.05.016. - DOI - PubMed
-
- Sultan I., Qaddoumi I., Yaser S., Rodriguez-Galindo C., Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. Journal of Clinical Oncology. 2009;27(20):3391–3397. doi: 10.1200/jco.2008.19.7483. - DOI - PubMed
LinkOut - more resources
Full Text Sources

