Modeling and Targeting Alzheimer's Disease With Organoids
- PMID: 32300301
- PMCID: PMC7145390
- DOI: 10.3389/fphar.2020.00396
Modeling and Targeting Alzheimer's Disease With Organoids
Abstract
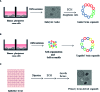
Human neurodegenerative diseases, such as Alzheimer's disease (AD), are not easily modeled in vitro due to the inaccessibility of brain tissue and the level of complexity required by existing cell culture systems. Three-dimensional (3D) brain organoid systems generated from human pluripotent stem cells (hPSCs) have demonstrated considerable potential in recapitulating key features of AD pathophysiology, such as amyloid plaque- and neurofibrillary tangle-like structures. A number of AD brain organoid models have also been used as platforms to assess the efficacy of pharmacological agents in disease progression. However, despite the fact that stem cell-derived brain organoids mimic early aspects of brain development, they fail to model complex cell-cell interactions pertaining to different regions of the human brain and aspects of natural processes such as cell differentiation and aging. Here, we review current advances and limitations accompanying several hPSC-derived organoid methodologies, as well as recent attempts to utilize them as therapeutic platforms. We additionally discuss comparative benefits and disadvantages of the various hPSC-derived organoid generation protocols and differentiation strategies. Lastly, we provide a comparison of hPSC-derived organoids to primary tissue-derived organoids, examining the future potential and advantages of both systems in modeling neurodegenerative disorders, especially AD.
Keywords: Alzheimer’s disease; disease modelling; hPSC-derived brain organoids; pharmacological treatments; primary tissue-derived organoids.
Copyright © 2020 Papaspyropoulos, Tsolaki, Foroglou and Pantazaki.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

