Fanconi Anemia Pathway: Mechanisms of Breast Cancer Predisposition Development and Potential Therapeutic Targets
- PMID: 32300589
- PMCID: PMC7142266
- DOI: 10.3389/fcell.2020.00160
Fanconi Anemia Pathway: Mechanisms of Breast Cancer Predisposition Development and Potential Therapeutic Targets
Abstract
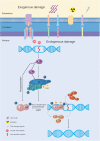
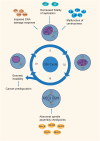
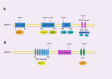
The maintenance of genomic stability is crucial for species survival, and its failure is closely associated with tumorigenesis. The Fanconi anemia (FA) pathway, involving 22 identified genes, plays a central role in repairing DNA interstrand cross-links. Importantly, a germline defect in any of these genes can cause Fanconi's anemia, a heterogeneous genetic disorder, characterized by congenital growth abnormalities, bone marrow failure, and predisposition to cancer. On the other hand, the breast cancer susceptibility genes, BRCA1 and BRCA2, also known as FANCS and FANCD1, respectively, are involved in the FA pathway; hence, researchers have studied the association between the FA pathway and cancer predisposition. Here, we mainly focused on and systematically reviewed the clinical and mechanistic implications of the predisposition of individuals with abnormalities in the FA pathway to cancer, especially breast cancer.
Keywords: Fanconi anemia; SNP; breast cancer; predisposition; susceptibility.
Copyright © 2020 Fang, Wu, Zhang, Liu and Zhang.
Figures
References
-
- Antoniou A. C., Pharoah P. D., Mcmullan G., Day N. E., Ponder B. A., Easton D. (2001). Evidence for further breast cancer susceptibility genes in addition to BRCA1 and BRCA2 in a population-based study. Genet. Epidemiol. 21 1–18. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

