Reweighting Randomized Controlled Trial Evidence to Better Reflect Real Life - A Case Study of the Innovative Medicines Initiative
- PMID: 32301116
- PMCID: PMC7540324
- DOI: 10.1002/cpt.1854
Reweighting Randomized Controlled Trial Evidence to Better Reflect Real Life - A Case Study of the Innovative Medicines Initiative
Abstract
Evidence from randomized controlled trials available for timely health technology assessments of new pharmacological treatments and regulatory decision making may not be generalizable to local patient populations, often resulting in decisions being made under uncertainty. In recent years, several reweighting approaches have been explored to address this important question of generalizability to a target population. We present a case study of the Innovative Medicines Initiative to illustrate the inverse propensity score reweighting methodology, which may allow us to estimate the expected treatment benefit if a clinical trial had been run in a broader real-world target population. We learned that identifying treatment effect modifiers, understanding and managing differences between patient characteristic data sets, and balancing the closeness of trial and target patient populations with effective sample size are key to successfully using this methodology and potentially mitigating some of this uncertainty around local decision making.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.H., A.B., D.F., K.B.W., A.G., J.J., and M.B. are employees and shareholders of Eli Lilly and Company. K.A., P.J., and Eli Lilly and Company are part of the Innovative Medicines Initiative GetReal consortium.
Figures
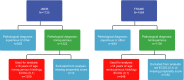
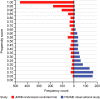

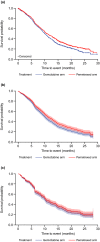
Comment in
-
The Promise, and Challenges, of Methods to Enhance the External Validity of Randomized Trial Results.Clin Pharmacol Ther. 2020 Dec;108(6):1132-1134. doi: 10.1002/cpt.1992. Epub 2020 Aug 8. Clin Pharmacol Ther. 2020. PMID: 32691848 Free PMC article. No abstract available.
Similar articles
-
Alternatives to Randomised Controlled Trials for the Poor, the Impatient, and When Evaluating Emerging Technologies.Eur J Vasc Endovasc Surg. 2019 Apr;57(4):598-599. doi: 10.1016/j.ejvs.2018.10.026. Epub 2018 Dec 1. Eur J Vasc Endovasc Surg. 2019. PMID: 30509892 No abstract available.
-
Five Steps to Successfully Implement and Evaluate Propensity Score Matching in Clinical Research Studies.Anesth Analg. 2018 Oct;127(4):1066-1073. doi: 10.1213/ANE.0000000000002787. Anesth Analg. 2018. PMID: 29324498
-
Quantitative decision-making in randomized Phase II studies with a time-to-event endpoint.J Biopharm Stat. 2019;29(1):189-202. doi: 10.1080/10543406.2018.1489400. Epub 2018 Jul 3. J Biopharm Stat. 2019. PMID: 29969380
-
Sample Size Estimation in Clinical Research: From Randomized Controlled Trials to Observational Studies.Chest. 2020 Jul;158(1S):S12-S20. doi: 10.1016/j.chest.2020.03.010. Chest. 2020. PMID: 32658647 Review.
-
Reliably basing conclusions on subgroups of randomized clinical trials.J Biopharm Stat. 2014;24(1):42-57. doi: 10.1080/10543406.2013.856020. J Biopharm Stat. 2014. PMID: 24392977 Review.
Cited by
-
Health technology assessment for the acute and preventive treatment of migraine: A position statement of the International Headache Society.Cephalalgia. 2021 Mar;41(3):279-293. doi: 10.1177/0333102421989247. Epub 2021 Jan 20. Cephalalgia. 2021. PMID: 33472427 Free PMC article.
-
The Promise, and Challenges, of Methods to Enhance the External Validity of Randomized Trial Results.Clin Pharmacol Ther. 2020 Dec;108(6):1132-1134. doi: 10.1002/cpt.1992. Epub 2020 Aug 8. Clin Pharmacol Ther. 2020. PMID: 32691848 Free PMC article. No abstract available.
-
Testing the "RCT augmentation" methodology: A trial simulation study to guide the broadening of trials eligibility criteria and inform on effectiveness.Contemp Clin Trials Commun. 2023 Apr 14;33:101142. doi: 10.1016/j.conctc.2023.101142. eCollection 2023 Jun. Contemp Clin Trials Commun. 2023. PMID: 37397428 Free PMC article.
-
Transporting Comparative Effectiveness Evidence Between Countries: Considerations for Health Technology Assessments.Pharmacoeconomics. 2024 Feb;42(2):165-176. doi: 10.1007/s40273-023-01323-1. Epub 2023 Oct 27. Pharmacoeconomics. 2024. PMID: 37891433 Free PMC article.
-
Generalizability of clinical trial efficacy results to a real-world population: An example in migraine prevention.J Manag Care Spec Pharm. 2023 Dec;29(12):1321-1330. doi: 10.18553/jmcp.2023.29.12.1321. J Manag Care Spec Pharm. 2023. PMID: 38058137 Free PMC article.
References
-
- UMC Utrecht . Welcome to GetReal. IMI GetReal <http://www.imi‐getreal.eu/> (2019). Accessed September 17, 2019.
-
- United Kingdom National Institute for Health and Care Excellence (NICE) . Guide to the processes of technology appraisal. Section 2.4.32 <https://www.nice.org.uk/Media/Default/About/what‐we‐do/NICE‐guidance/NIC...> (April 2018). Accessed September 17, 2019. - PubMed
-
- United Kingdom National Institute for Health and Care Excellence (NICE) . Guide to methods of technology appraisal 2013: process and methods. Sections 3.2.2 and 3.3.3 <https://www.nice.org.uk/process/pmg9/resources/guide‐to‐the‐methods‐of‐t...> (April 2013). Accessed September 17, 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

