Rapid response
- PMID: 32301437
- PMCID: PMC7164951
- DOI: 10.7554/eLife.57105
Rapid response
Abstract
Extremely short X-ray pulses from a free-electron laser are helping to clarify how phytochromes respond to light, but puzzles remain.
Keywords: Deinococcus radiodurans; SFX; free-electron laser; initial photoresponse; molecular biophysics; phytochromes; structural biology.
© 2020, Hughes.
Conflict of interest statement
JH No competing interests declared
Figures
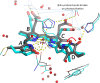
Comment on
-
The primary structural photoresponse of phytochrome proteins captured by a femtosecond X-ray laser.Elife. 2020 Mar 31;9:e53514. doi: 10.7554/eLife.53514. Elife. 2020. PMID: 32228856 Free PMC article.
References
-
- Claesson E, Wahlgren WY, Takala H, Pandey S, Castillon L, Kuznetsova V, Henry L, Panman M, Carrillo M, Kübel J, Nanekar R, Isaksson L, Nimmrich A, Cellini A, Morozov D, Maj M, Kurttila M, Bosman R, Nango E, Tanaka R, Tanaka T, Fangjia L, Iwata S, Owada S, Moffat K, Groenhof G, Stojković EA, Ihalainen JA, Schmidt M, Westenhoff S. The primary structural photoresponse of phytochrome proteins captured by a femtosecond X-ray laser. eLife. 2020;9:e53514. doi: 10.7554/eLife.53514. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

