The cellular expression and proteolytic processing of the amyloid precursor protein is independent of TDP-43
- PMID: 32301481
- PMCID: PMC7189496
- DOI: 10.1042/BSR20200435
The cellular expression and proteolytic processing of the amyloid precursor protein is independent of TDP-43
Abstract
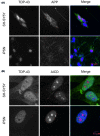
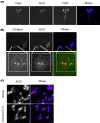
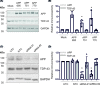
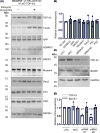
Alzheimer's disease (AD) is a neurodegenerative condition, of which one of the cardinal pathological hallmarks is the extracellular accumulation of amyloid β (Aβ) peptides. These peptides are generated via proteolysis of the amyloid precursor protein (APP), in a manner dependent on the β-secretase, BACE1 and the multicomponent γ-secretase complex. Recent data also suggest a contributory role in AD of transactive response DNA binding protein 43 (TDP-43). There is little insight into a possible mechanism linking TDP-43 and APP processing. To this end, we used cultured human neuronal cells to investigate the ability of TDP-43 to interact with APP and modulate its proteolytic processing. Immunocytochemistry showed TDP-43 to be spatially segregated from both the extranuclear APP holoprotein and its nuclear C-terminal fragment. The latter (APP intracellular domain) was shown to predominantly localise to nucleoli, from which TDP-43 was excluded. Furthermore, neither overexpression of each of the APP isoforms nor siRNA-mediated knockdown of APP had any effect on TDP-43 expression. Doxycycline-stimulated overexpression of TDP-43 was explored in an inducible cell line. Overexpression of TDP-43 had no effect on expression of the APP holoprotein, nor any of the key proteins involved in its proteolysis. Furthermore, increased TDP-43 expression had no effect on BACE1 enzymatic activity or immunoreactivity of Aβ1-40, Aβ1-42 or the Aβ1-40:Aβ1-42 ratio. Also, siRNA-mediated knockdown of TDP-43 had no effect on BACE1 immunoreactivity. Taken together, these data indicate that TDP-43 function and/or dysfunction in AD is likely independent from dysregulation of APP expression and proteolytic processing and Aβ generation.
Keywords: Alzheimers disease; Amyloid precursor protein; BACE1; Gene regulation; TDP-43; proteolysis.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

