Yolk-sac-derived macrophages progressively expand in the mouse kidney with age
- PMID: 32301704
- PMCID: PMC7205460
- DOI: 10.7554/eLife.51756
Yolk-sac-derived macrophages progressively expand in the mouse kidney with age
Abstract
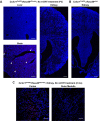
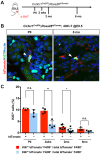
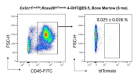
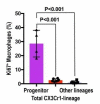
Renal macrophages represent a highly heterogeneous and specialized population of myeloid cells with mixed developmental origins from the yolk-sac and hematopoietic stem cells (HSC). They promote both injury and repair by regulating inflammation, angiogenesis, and tissue remodeling. Recent reports highlight differential roles for ontogenically distinct renal macrophage populations in disease. However, little is known about how these populations change over time in normal, uninjured kidneys. Prior reports demonstrated a high proportion of HSC-derived macrophages in the young adult kidney. Unexpectedly, using genetic fate-mapping and parabiosis studies, we found that yolk-sac-derived macrophages progressively expand in number with age and become a major contributor to the renal macrophage population in older mice. This chronological shift in macrophage composition involves local cellular proliferation and recruitment from circulating progenitors and may contribute to the distinct immune responses, limited reparative capacity, and increased disease susceptibility of kidneys in the elderly population.
Keywords: aging; human biology; immunology; inflammation; kidney; macrophage ontogeny; medicine; mouse.
Plain language summary
Older people are more likely to develop kidney disease, which increases their risk of having other conditions such as a heart attack or stroke and, in some cases, can lead to their death. Older kidneys are less able to repair themselves after an injury, which may help explain why aging contributes to kidney disease. Another possibility is that older kidneys are more susceptible to excessive inflammation. Learning more about the processes that lead to kidney inflammation in older people might lead to better ways to prevent or treat their kidney disease. Immune cells called macrophages help protect the body from injury and disease. They do this by triggering inflammation, which aides healing. Too much inflammation can be harmful though, making macrophages a prime suspect in age-related kidney harm. Studying these immune cells in the kidney and how they change over the lifespan could help scientists to better understand age-related kidney disease. Now, Ide, Yahara et al. show that one type of macrophage is better at multiplying in older kidneys. In the experiments, mice were genetically engineered to make a fluorescent red protein in one kind of macrophage. This allowed Ide, Yahara et al. to track these immune cells as the mice aged. The experiments showed that this subgroup of cells is first produced when the mice are embryos. They stay in the mouse kidneys into adulthood, and are so prolific that, over time, they eventually become the most common macrophage in older kidneys. The fact that one type of embryonically derived macrophage takes over with age may explain the increased inflammation and reduced repair capacity seen in aging kidneys. More studies will help scientists to understand how these particular cells contribute to age-related changes in susceptibility to kidney disease.
© 2020, Ide et al.
Conflict of interest statement
SI, YY, YK, SS, KI, AW, SX, JP, SC, MS, BA, TS No competing interests declared
Figures








References
-
- Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nature Immunology. 2014;15:929–937. doi: 10.1038/ni.2967. - DOI - PMC - PubMed
-
- Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, Steers NJ, Mack M, Joshi A, Guilliams M, Mowat AM, Geissmann F, Jenkins SJ. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nature Communications. 2016;7:ncomms11852. doi: 10.1038/ncomms11852. - DOI - PMC - PubMed
-
- Brähler S, Zinselmeyer BH, Raju S, Nitschke M, Suleiman H, Saunders BT, Johnson MW, Böhner AMC, Viehmann SF, Theisen DJ, Kretzer NM, Briseño CG, Zaitsev K, Ornatsky O, Chang Q, Carrero JA, Kopp JB, Artyomov MN, Kurts C, Murphy KM, Miner JH, Shaw AS. Opposing roles of dendritic cell subsets in experimental GN. Journal of the American Society of Nephrology. 2018;29:138–154. doi: 10.1681/ASN.2017030270. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

