Transcription Factor 4 Regulates the Regeneration of Corneal Endothelial Cells
- PMID: 32301972
- PMCID: PMC7401711
- DOI: 10.1167/iovs.61.4.21
Transcription Factor 4 Regulates the Regeneration of Corneal Endothelial Cells
Retraction in
-
Retraction.Invest Ophthalmol Vis Sci. 2020 Sep 1;61(11):6. doi: 10.1167/iovs.61.11.6. Invest Ophthalmol Vis Sci. 2020. PMID: 32882012 Free PMC article. No abstract available.
Abstract
Purpose: Human corneal endothelial cells (hCECs) have limited regenerative capacity in vivo. Reduced hCEC density results in bullous keratopathy requiring corneal transplantation. This study reveals the role of transcription factor 4 (TCF4) in hCEC diseases and suggests that TCF4 may be a molecular target for hCEC regeneration.
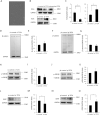
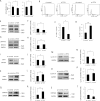
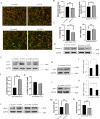
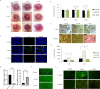
Methods: Cell shape, cell proliferation rates, and proliferation-associated proteins were evaluated in normal or senescent hCECs. TCF4 was blocked by siRNA (si-TCF4) or activated using clustered regularly interspaced short palindromic repeats (CRISPR)/dCas9 activation systems (pl-TCF4). The corneal endothelium of six-week-old Sprague-Dawley (SD) rats was transfected by electroporation followed by cryoinjury.
Results: Cell proliferation rates and TCF4 levels were reduced in senescent cells. TCF4 CRISPR activation enhanced corneal endothelial wound healing. TCF4 regulated mitochondrial functions including mitochondrial membrane potential, mitochondrial superoxide levels, and energy production. The percentage of cells in the S-phase was reduced with si-TCF4 and increased with pl-TCF4. Cell proliferation and cell cycle-associated proteins were regulated by TCF4. Autophagy was induced by si-TCF4. In vivo transfection of CRISPR/dCas9 activation systems (a-TCF4) induced regeneration of corneal endothelium.
Conclusions: Corneal endothelial diseases are associated with TCF4 reduction; TCF4 may be a potential target for hCEC diseases. Gene therapy using TCF4 CRISPR/dCas9 may be an effective treatment for hCEC diseases.
Conflict of interest statement
Disclosure:
Figures

References
-
- Waring GO 3rd, Bourne WM, Edelhauser HF, Kenyon KR. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology. 1982; 89: 531–590. - PubMed
-
- Whikehart DR. The inhibition of sodium, potassium-stimulated ATPase and corneal swelling: the role played by polyols. J Am Optom Assoc. 1995; 66: 331–333. - PubMed
-
- Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci. 1977; 16: 597–613. - PubMed
-
- Baratz KH, Tosakulwong N, Ryu E, et al. .. E2-2 protein and Fuchs's corneal dystrophy. N Engl J Med. 2010; 363: 1016–1024. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

