Relaxed constraint and functional divergence of the progesterone receptor (PGR) in the human stem-lineage
- PMID: 32302297
- PMCID: PMC7190170
- DOI: 10.1371/journal.pgen.1008666
Relaxed constraint and functional divergence of the progesterone receptor (PGR) in the human stem-lineage
Abstract
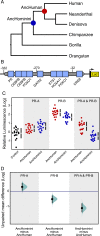
The steroid hormone progesterone, acting through the progesterone receptor (PR), a ligand-activated DNA-binding transcription factor, plays an essential role in regulating nearly every aspect of female reproductive biology. While many reproductive traits regulated by PR are conserved in mammals, Catarrhine primates evolved several derived traits including spontaneous decidualization, menstruation, and a divergent (and unknown) parturition signal, suggesting that PR may also have evolved divergent functions in Catarrhines. There is conflicting evidence, however, whether the progesterone receptor gene (PGR) was positively selected in the human lineage. Here we show that PGR evolved rapidly in the human stem-lineage (as well as other Catarrhine primates), which likely reflects an episode of relaxed selection intensity rather than positive selection. Coincident with the episode of relaxed selection intensity, ancestral sequence resurrection and functional tests indicate that the major human PR isoforms (PR-A and PR-B) evolved divergent functions in the human stem-lineage. These results suggest that the regulation of progesterone signaling by PR-A and PR-B may also have diverged in the human lineage and that non-human animal models of progesterone signaling may not faithfully recapitulate human biology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


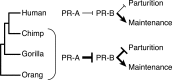
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

