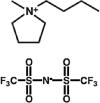
Investigating the potential use of an ionic liquid (1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide) as an anti-fungal treatment against the amphibian chytrid fungus, Batrachochytrium dendrobatidis
- PMID: 32302369
- PMCID: PMC7164615
- DOI: 10.1371/journal.pone.0231811
Investigating the potential use of an ionic liquid (1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide) as an anti-fungal treatment against the amphibian chytrid fungus, Batrachochytrium dendrobatidis
Abstract
The disease chytridiomycosis, caused by the pathogenic chytrid fungus, Batrachochytrium dendrobatidis (Bd), has contributed to global amphibian declines. Bd infects the keratinized epidermal tissue in amphibians and causes hyperkeratosis and excessive skin shedding. In individuals of susceptible species, the regulatory function of the amphibian's skin is disrupted resulting in an electrolyte depletion, osmotic imbalance, and eventually death. Safe and effective treatments for chytridiomycosis are urgently needed to control chytrid fungal infections and stabilize populations of endangered amphibian species in captivity and in the wild. Currently, the most widely used anti-Bd treatment is itraconazole. Preparations of itraconazole formulated for amphibian use has proved effective, but treatment involves short baths over seven to ten days, a process which is logistically challenging, stressful, and causes long-term health effects. Here, we explore a novel anti-fungal therapeutic using a single application of the ionic liquid, 1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide (BMP-NTf2), for the treatment of chytridiomycosis. BMP-NTf2 was found be effective at killing Bd in vitro at low concentrations (1:1000 dilution). We tested BMP-NTf2 in vivo on two amphibian species, one that is relatively tolerant of chytridiomycosis (Pseudacris regilla) and one that is highly susceptible (Dendrobates tinctorius). A toxicity trial revealed a surprising interaction between Bd infection status and the impact of BMP-NTf2 on D. tinctorius survival. Uninfected D. tinctorius tolerated BMP-NTf2 (mean ± SE; 96.01 ± 9.00 μl/g), such that only 1 out of 30 frogs died following treatment (at a dose of 156.95 μL/g), whereas, a lower dose (mean ± SE; 97.45 ± 3.52 μL/g) was not tolerated by Bd-infected D. tinctorius, where 15 of 23 frogs died shortly upon BMP-NTf2 application. Those that tolerated the BMP-NTf2 application did not exhibit Bd clearance. Thus, BMP-NTf2 application, under the conditions tested here, is not a suitable option for clearing Bd infection in D. tinctorius. However, different results were obtained for P. regilla. Two topical applications of BMP-NTf2 on Bd-infected P. regilla (using a lower BMP-NTf2 dose than on D. tinctorius, mean ± SE; 9.42 ± 1.43 μL/g) reduced Bd growth, although the effect was lower than that obtained by daily doses of itracanozole (50% frogs exhibited complete clearance on day 16 vs. 100% for itracanozole). Our findings suggest that BMP-NTf2 has the potential to treat Bd infection, however the effect depends on several parameters. Further optimization of dose and schedule are needed before BMP-NTf2 can be considered as a safe and effective alternative to more conventional antifungal agents, such as itraconazole.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Longcore JE, Pessier AP, Nichols DK. Batrachochytrium Dendrobatidis gen. et sp. nov., a Chytrid Pathogenic to amphibians. Mycologia. 1999;91: 219–227.
-
- Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth. 2007;4: 125–134. 10.1007/s10393-007-0093-5 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

