PNOCARC Neurons Promote Hyperphagia and Obesity upon High-Fat-Diet Feeding
- PMID: 32302532
- PMCID: PMC7303947
- DOI: 10.1016/j.neuron.2020.03.022
PNOCARC Neurons Promote Hyperphagia and Obesity upon High-Fat-Diet Feeding
Abstract
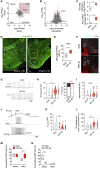
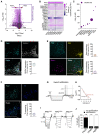
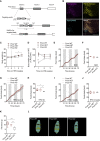
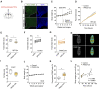
Calorie-rich diets induce hyperphagia and promote obesity, although the underlying mechanisms remain poorly defined. We find that short-term high-fat-diet (HFD) feeding of mice activates prepronociceptin (PNOC)-expressing neurons in the arcuate nucleus of the hypothalamus (ARC). PNOCARC neurons represent a previously unrecognized GABAergic population of ARC neurons distinct from well-defined feeding regulatory AgRP or POMC neurons. PNOCARC neurons arborize densely in the ARC and provide inhibitory synaptic input to nearby anorexigenic POMC neurons. Optogenetic activation of PNOCARC neurons in the ARC and their projections to the bed nucleus of the stria terminalis promotes feeding. Selective ablation of these cells promotes the activation of POMC neurons upon HFD exposure, reduces feeding, and protects from obesity, but it does not affect food intake or body weight under normal chow consumption. We characterize PNOCARC neurons as a novel ARC neuron population activated upon palatable food consumption to promote hyperphagia.
Keywords: PNOC neurons; acute high-fat-diet feeding; arcuate nucleus; food intake; hypothalamus; neuropeptide; nociceptin; obesity; orphanin FQ; prepronociceptin.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




References
-
- Backes H., Walberer M., Endepols H., Neumaier B., Graf R., Wienhard K., Mies G. Whiskers area as extracerebral reference tissue for quantification of rat brain metabolism using (18)F-FDG PET: application to focal cerebral ischemia. J. Nucl. Med. 2011;52:1252–1260. - PubMed
-
- Balthasar N., Coppari R., McMinn J., Liu S.M., Lee C.E., Tang V., Kenny C.D., McGovern R.A., Chua S.C., Jr., Elmquist J.K., Lowell B.B. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

