Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage
- PMID: 32302590
- PMCID: PMC7237863
- DOI: 10.1016/j.cell.2020.03.052
Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage
Abstract
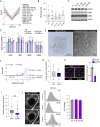
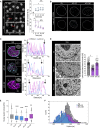
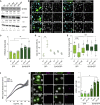
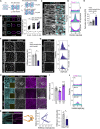
Tissue homeostasis requires maintenance of functional integrity under stress. A central source of stress is mechanical force that acts on cells, their nuclei, and chromatin, but how the genome is protected against mechanical stress is unclear. We show that mechanical stretch deforms the nucleus, which cells initially counteract via a calcium-dependent nuclear softening driven by loss of H3K9me3-marked heterochromatin. The resulting changes in chromatin rheology and architecture are required to insulate genetic material from mechanical force. Failure to mount this nuclear mechanoresponse results in DNA damage. Persistent, high-amplitude stretch induces supracellular alignment of tissue to redistribute mechanical energy before it reaches the nucleus. This tissue-scale mechanoadaptation functions through a separate pathway mediated by cell-cell contacts and allows cells/tissues to switch off nuclear mechanotransduction to restore initial chromatin state. Our work identifies an unconventional role of chromatin in altering its own mechanical state to maintain genome integrity in response to deformation.
Keywords: DNA damage; chromatin; heterochromatin; mechanoprotection; mechanotransduction; nuclear architecture; nuclear lamina; nuclear mechanics; stem cells.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures










Comment in
-
Feeling Stressed? Piezo1-Mediated Loss of Heterochromatin Buys Time for Long-Term Adaptation.Cell. 2020 May 14;181(4):760-762. doi: 10.1016/j.cell.2020.04.041. Cell. 2020. PMID: 32413297
-
Genome protection on the move.Cardiovasc Res. 2020 Aug 1;116(10):e109-e111. doi: 10.1093/cvr/cvaa189. Cardiovasc Res. 2020. PMID: 32707575 No abstract available.
References
-
- Adding L.C., Bannenberg G.L., Gustafsson L.E. Basic experimental studies and clinical aspects of gadolinium salts and chelates. Cardiovasc. Drug Rev. 2001;19:41–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

