Antibody development for preventing the human respiratory syncytial virus pathology
- PMID: 32303184
- PMCID: PMC7164255
- DOI: 10.1186/s10020-020-00162-6
Antibody development for preventing the human respiratory syncytial virus pathology
Abstract
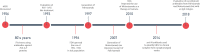
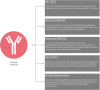
Human respiratory syncytial virus (hRSV) is the most important etiological agent causing hospitalizations associated with respiratory diseases in children under 5 years of age as well as the elderly, newborns and premature children are the most affected populations. This viral infection can be associated with various symptoms, such as fever, coughing, wheezing, and even pneumonia and bronchiolitis. Due to its severe symptoms, the need for mechanical ventilation is not uncommon in clinical practice. Additionally, alterations in the central nervous system -such as seizures, encephalopathy and encephalitis- have been associated with cases of hRSV-infections. Furthermore, the absence of effective vaccines or therapies against hRSV leads to elevated expenditures by the public health system and increased mortality rates for the high-risk population. Along these lines, vaccines and therapies can elicit different responses to this virus. While hRSV vaccine candidates seek to promote an active immune response associated with the achievement of immunological memory, other therapies -such as the administration of antibodies- provide a protective environment, although they do not trigger the activation of the immune system and therefore do not promote an immunological memory. An interesting approach to vaccination is the use of virus-neutralizing antibodies, which inhibit the entry of the pathogen into the host cells, therefore impairing the capacity of the virus to replicate. Currently, the most common molecule targeted for antibody design against hRSV is the F protein of this virus. However, other molecular components of the virus -such as the G or the N hRSV proteins- have also been explored as potential targets for the control of this disease. Currently, palivizumab is the only monoclonal antibody approved for human use. However, studies in humans have shown a protective effect only after the administration of at least 3 to 5 doses, due to the stability of this vaccine. Furthermore, other studies suggest that palivizumab only has an effectiveness close to 50% in high-risk infants. In this work, we will review different strategies addressed for the use of antibodies in a prophylactic or therapeutic context and their ability to prevent the symptoms caused by hRSV infection of the airways, as well as in other tissues such as the CNS.
Keywords: Antibodies; Human orthopneumovirus; Passive transference; Prophylaxis; Therapy; hRSV.
Conflict of interest statement
The authors declare the following possible conflict of interest: “Monoclonal Antibody specific against the antigen N of the Human Syncitial Respiratory Virus (VRSH), useful for the treatment of infection, its detection and diagnosis.PCT / CL2018 / 050079, date of presentation September 2018”.
Figures
References
-
- Aliprantis A, et al. 1971. A Randomized, Double-Blind, Placebo-Controlled Trial to Assess the Safety and Tolerability of a Respiratory Syncytial Virus (RSV) Neutralizing Monoclonal Antibody (MK-1654) in Healthy Subjects. Open Forum Infect Dis. 2018;5(suppl_1):S572. doi: 10.1093/ofid/ofy210.1627. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

