Clinical efficacy, safety and tolerability of Aliskiren Monotherapy (AM): an umbrella review of systematic reviews
- PMID: 32303191
- PMCID: PMC7164287
- DOI: 10.1186/s12872-020-01442-z
Clinical efficacy, safety and tolerability of Aliskiren Monotherapy (AM): an umbrella review of systematic reviews
Abstract
Background: Aliskiren is a newly developed drug. Its role in lowering BP has been recognized. However, the role of aliskiren in treating heart and renal diseases are still controversial.
Objective: To evaluate the existing evidence about clinical efficacy, safety and tolerability of aliskiren monotherapy (AM).
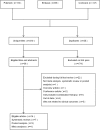
Methods: An umbrella review of systematic reviews of interventional studies. We searched Pubmed, Embase and Cochrane Library up to June 2019. Two reviewers applied inclusion criteria to the select potential articles independently. The extract and analyze of accessible data were did by two reviewers independently too. Discrepancies were resolved with discussion or the arbitration of the third author.
Results: Eventually, our review identified 14 eligible studies. Results showed that for essential hypertension patients, aliskiren showed a great superiority over placebo in BP reduction, BP response rate and BP control rate. Aliskiren and placebo, ARBs or ACEIs showed no difference in the number or extent of adverse events. For heart failure patients, AM did not reduce BNP levels (SMD -0.08, - 0.31 to 0.15) or mortality rate (RR 0.76, 0.32 to 1.80), but it decreased NT-proBNP (SMD -0.12, - 0.21 to - 0.03) and PRA levels (SMD 0.52, 0.30 to 0.75), increased PRC levels (SMD -0.66, - 0.8 to - 0.44). For patients who are suffered from hypertension and diabetes and/or nephropathy or albuminuria at the same time, aliskiren produced no significant effects (RR 0.97, 0.81 to 1.16).
Conclusion: We found solid evidence to support the benefits of aliskiren in the treatment of essential hypertension, aliskiren can produce significant effects in lowering BP and reliable safety. However, the effects of aliskiren in cardiovascular and renal outcomes were insignificant.
Trial registration: Study has been registered in PROSPERO (CRD42019142141).
Keywords: Aliskiren; Clinical effectiveness; Monotherapy; Safety.
Conflict of interest statement
All our authors report no conflicts of interest in this work.
Figures
References
-
- Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. - DOI - PubMed
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/s0140-6736(12)61729-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials