Pediatric bithalamic gliomas have a distinct epigenetic signature and frequent EGFR exon 20 insertions resulting in potential sensitivity to targeted kinase inhibition
- PMID: 32303840
- PMCID: PMC7792550
- DOI: 10.1007/s00401-020-02155-5
Pediatric bithalamic gliomas have a distinct epigenetic signature and frequent EGFR exon 20 insertions resulting in potential sensitivity to targeted kinase inhibition
Abstract
Brain tumors are the most common solid tumors of childhood, and the genetic drivers and optimal therapeutic strategies for many of the different subtypes remain unknown. Here, we identify that bithalamic gliomas harbor frequent mutations in the EGFR oncogene, only rare histone H3 mutation (in contrast to their unilateral counterparts), and a distinct genome-wide DNA methylation profile compared to all other glioma subtypes studied to date. These EGFR mutations are either small in-frame insertions within exon 20 (intracellular tyrosine kinase domain) or missense mutations within exon 7 (extracellular ligand-binding domain) that occur in the absence of accompanying gene amplification. We find these EGFR mutations are oncogenic in primary astrocyte models and confer sensitivity to specific tyrosine kinase inhibitors dependent on location within the kinase domain or extracellular domain. We initiated treatment with targeted kinase inhibitors in four children whose tumors harbor EGFR mutations with encouraging results. This study identifies a promising genomically-tailored therapeutic strategy for bithalamic gliomas, a lethal and genetically distinct brain tumor of childhood.
Keywords: Afatinib; Bithalamic glioma; Diffuse midline glioma; EGFR; Erlotinib; Histone H3; Molecular neuropathology; Osimertinib; Pediatric cancer; Trametinib; Tyrosine kinase inhibitor.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
S.J. Allen is a current employee of Illumina, Inc. No potential conflicts of interest were disclosed by any of the other authors.
Figures
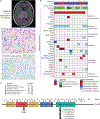
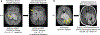

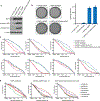
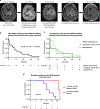
References
-
- Benbir G, Sayilir I, Oz B, et al. (2008) Bilateral thalamic glioma. A case report. J Neurol Sci 25:301–5.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

