Pharmacokinetic Profile of Gilteritinib: A Novel FLT-3 Tyrosine Kinase Inhibitor
- PMID: 32304015
- PMCID: PMC7550323
- DOI: 10.1007/s40262-020-00888-w
Pharmacokinetic Profile of Gilteritinib: A Novel FLT-3 Tyrosine Kinase Inhibitor
Erratum in
-
Correction to: Pharmacokinetic Profile of Gilteritinib: A Novel FLT-3 Tyrosine Kinase Inhibitor.Clin Pharmacokinet. 2021 Sep;60(9):1251. doi: 10.1007/s40262-021-01060-8. Clin Pharmacokinet. 2021. PMID: 34297319 Free PMC article. No abstract available.
Abstract
Background and objective: Gilteritinib is a novel, highly selective tyrosine kinase inhibitor approved in the USA, Canada, Europe, Brazil, Korea, and Japan for the treatment of FLT3 mutation-positive acute myeloid leukemia. This article describes the clinical pharmacokinetic profile of gilteritinib.
Methods: The pharmacokinetic profile of gilteritinib was assessed from five clinical studies.
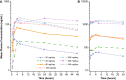
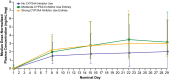
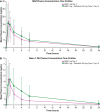
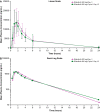
Results: Dose-proportional pharmacokinetics was observed following once-daily gilteritinib administration (dose range 20-450 mg). Median maximum concentration was reached 2-6 h following single and repeat dosing of gilteritinib; mean elimination half-life was 113 h. Elimination was primarily via feces. Exposure to gilteritinib was comparable under fasted and fed conditions. Gilteritinib is primarily metabolized via cytochrome P450 (CYP) 3A4; coadministration of gilteritinib with itraconazole (a strong P-glycoprotein inhibitor and CYP3A4 inhibitor) or rifampicin (a strong P-glycoprotein inducer and CYP3A inducer) significantly affected the gilteritinib pharmacokinetic profile. No clinically relevant interactions were observed when gilteritinib was coadministered with midazolam (a CYP3A4 substrate) or cephalexin (a multidrug and toxin extrusion 1 substrate). Unbound gilteritinib exposure was similar between subjects with hepatic impairment and normal hepatic function.
Conclusions: Gilteritinib exhibits a dose-proportional pharmacokinetic profile in healthy subjects and in patients with relapsed/refractory acute myeloid leukemia. Gilteritinib exposure is not significantly affected by food. Moderate-to-strong CYP3A inhibitors demonstrated a significant effect on gilteritinib exposure. Coadministration of gilteritinib with CYP3A4 or multidrug and toxin extrusion 1 substrates did not impact substrate concentrations. Unbound gilteritinib was comparable between subjects with hepatic impairment and normal hepatic function; dose adjustment is not warranted for patients with hepatic impairment.
Clinical trial registration: NCT02014558, NCT02456883, NCT02571816.
Conflict of interest statement
Angela Joubert James, Melanie Patton, Chaofeng Liu, Selina Moy, and Erkut Bahceci are employees of Astellas Pharma, US Inc. Zheng Lu was an employee of Astellas Pharma US, Inc. during the time of the study and development of the manuscript. Takeshi Kadokura and Kinya Souda are employees of Astellas Pharma, Inc. Catherine C. Smith received research grants from Astellas Pharma US, Inc. Mark Litzow has no conflicts of interest that are directly relevant to the content of this article. Alexander E. Perl has received funding, honoraria, or travel reimbursement from Astellas, Daiichi-Sankyo, Arog, Novartis, Pfizer, Actinium Pharmaceuticals, Jazz Pharmaceuticals, Takeda, AbbVie, NewLink Genetics, Asana Biosciences, and Seattle Genetics. Jessica K. Altman has received funding, honoraria, or travel reimbursement from AbbVie, Agios, Ariad, Astellas, Bayer, BioSight, BMS, Boeringer Ingelheim, Cancer Expert Now, Celator, Celgene, Daiichi Sankyo, Epizyme, France Foundation, FujiFilm, Genentech, Glycomimetics, GSK, Incyte, Janssen Pharmaceuticals, Novartis, PeerView, Pfizer, prIME Oncology, Syros, and Theradex. Dale Shepard has received funding, honoraria, or travel reimbursement from Celgene, Sanofi, AstraZeneca, Ipsen, Amgen, Genentech, Eli Lilly, Bayer, Leap, Alkermes, Aduro, Halozyme, Ignyta, BMS, Pfizer, Kinex, and Corvus. Mark J. Levis has received funding or personal fees from Astellas, Novartis, Daiichi-Sankyo, and FujiFilm.
Figures



References
-
- The American Cancer Society . Cancer facts & figures 2018. Atlanta (GA): American Cancer Society; 2018.
-
- Ben-Batalla I, Schultze A, Wroblewski M, Erdmann R, Heuser M, Waizenegger JS, et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood. 2013;122(14):2443–2452. doi: 10.1182/blood-2013-03-491431. - DOI - PubMed
-
- Park IK, Mishra A, Chandler J, Whitman SP, Marcucci G, Caligiuri MA. Inhibition of the receptor tyrosine kinase Axl impedes activation of the FLT3 internal tandem duplication in human acute myeloid leukemia: implications for Axl as a potential therapeutic target. Blood. 2013;121(11):2064–2073. doi: 10.1182/blood-2012-07-444018. - DOI - PMC - PubMed
-
- Gunawardane RN, Nepomuceno RR, Rooks AM, Hunt JP, Ricono JM, Belli B, et al. Transient exposure to quizartinib mediates sustained inhibition of FLT3 signaling while specifically inducing apoptosis in FLT3-activated leukemia cells. Mol Cancer Ther. 2013;12(4):438–447. doi: 10.1158/1535-7163.MCT-12-0305. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

