Replacement and desmoplastic histopathological growth patterns in cutaneous melanoma liver metastases: frequency, characteristics, and robust prognostic value
- PMID: 32304183
- PMCID: PMC7339161
- DOI: 10.1002/cjp2.161
Replacement and desmoplastic histopathological growth patterns in cutaneous melanoma liver metastases: frequency, characteristics, and robust prognostic value
Abstract
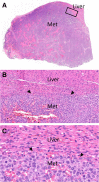
Among visceral metastatic sites, cutaneous melanoma (CM) metastasises initially to the liver in ~14-20% of cases. Liver metastases in CM patients are associated with both poor prognosis and poor response to immunotherapy. Histopathological growth patterns (HGPs) of liver metastases of the replacement and desmoplastic type, particularly from colorectal cancer and uveal melanoma (UM), may impart valuable biological and prognostic information. Here, we have studied HGP in 43 CM liver metastases resected from 42 CM patients along with other prognostic factors from three institutions. The HGPs (replacement, desmoplastic, pushing) were scored at the metastasis-liver interface with two algorithms: (1) 100% desmoplastic growth pattern (dHGP) and any (≥1%) replacement pattern (any-rHGP) and (2) >50% dHGP, >50% rHGP or mixed (<50% dHGP and/or rHGP, pushing HGP). For 1 patient with 2 metastases, an average was taken to obtain 1 final HGP yielding 42 observations from 42 patients. 22 cases (52%) had 100% dHGP whereas 20 (48%) had any replacement. Cases with rHGP demonstrated vascular co-option/angiotropism. With the development of liver metastasis, only rHGP (both algorithms), male gender and positive resection margins predicted diminished overall survival (p = 0.00099 and p = 0.0015; p = 0.034 and p = 0.024 respectively). On multivariate analysis, only HGP remained significant. 7 of 42 (17%) patients were alive with disease and 21 (50%) died with follow-up after liver metastases ranging from 1.8 to 42.2 months (mean: 20.4 months, median: 19.0 months). 14 (33%) patients with previously-treated metastatic disease had no evidence of disease at last follow up. In conclusion, we report for the first time replacement and desmoplastic HGPs in CM liver metastases and their prognostic value, as in UM and other solid cancers. Of particular importance, any rHGP significantly predicted diminished overall survival while 100% dHGP correlated with increased survival. These results contribute to a better understanding of the biology of CM liver metastases and potentially may be utilised in managing patients with these metastases.
Keywords: angiotropism; cutaneous melanoma; desmoplastic; extravascular migratory metastasis; histopathological growth patterns; liver; metastasis; pericytic mimicry; replacement; vascular co-option.
© 2020 The Authors. The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland and John Wiley & Sons Ltd.
Figures




References
-
- Pawlik TM, Zorzi D, Abdalla EK, et al Hepatic resection for metastatic melanoma: distinct patterns of recurrence and prognosis for ocular versus cutaneous disease. Ann Surg Oncol 2006; 13: 712–720. - PubMed
-
- Agarwala SS, Eggermont AM, O'Day S, et al Metastatic melanoma to the liver: a contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer 2014; 120: 781–789. - PubMed
-
- Tas F, Erturk K. Recurrence behavior in early‐stage cutaneous melanoma: pattern, timing, survival, and influencing factors. Melanoma Res 2017; 27: 134–139. - PubMed
-
- Marcoval J, Ferreres JR, Martín C, et al Patterns of visceral metastasis in cutaneous melanoma: a descriptive study. Actas Dermosifiliogr 2013; 104: 593–597. - PubMed
-
- Aubin JM, Rekman J, Vandenbroucke‐Menu F, et al Systematic review and meta‐analysis of liver resection for metastatic melanoma. Br J Surg 2013; 100: 1138–1147. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

