Fucoidan functionalization on poly(vinyl alcohol) hydrogels for improved endothelialization and hemocompatibility
- PMID: 32304872
- PMCID: PMC7748769
- DOI: 10.1016/j.biomaterials.2020.120011
Fucoidan functionalization on poly(vinyl alcohol) hydrogels for improved endothelialization and hemocompatibility
Abstract
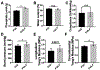
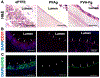
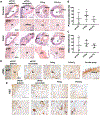
The performance of clinical synthetic small diameter vascular grafts remains disappointing due to the fast occlusion caused by thrombosis and intimal hyperplasia formation. Poly(vinyl alcohol) (PVA) hydrogels have tunable mechanical properties and a low thrombogenic surface, which suggests its potential value as a small diameter vascular graft material. However, PVA does not support cell adhesion and thus requires surface modification to encourage endothelialization. This study presents a modification of PVA with fucoidan. Fucoidan is a sulfated polysaccharide with anticoagulant and antithrombotic properties, which was shown to potentially increase endothelial cell adhesion and proliferation. By mixing fucoidan with PVA and co-crosslinked by sodium trimetaphosphate (STMP), the modification was achieved without sacrificing mechanical properties. Endothelial cell adhesion and monolayer function were significantly enhanced by the fucoidan modification. In vitro and ex-vivo studies showed low platelet adhesion and activation and decreased thrombin generation with fucoidan modified PVA. The modification proved to be compatible with gamma sterilization. In vivo evaluation of fucoidan modified PVA grafts in rabbits exhibited increased patency rate, endothelialization, and reduced intimal hyperplasia formation. The fucoidan modification presented here benefited the development of PVA vascular grafts and can be adapted to other blood contacting surfaces.
Keywords: End-to-side anastomosis; Endothelialization; Fucoidan; Hemocompatibility; Rabbit carotid artery; Small diameter vascular graft.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



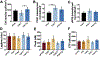


Similar articles
-
In vitro and ex vivo hemocompatibility of off-the-shelf modified poly(vinyl alcohol) vascular grafts.Acta Biomater. 2015 Oct;25:97-108. doi: 10.1016/j.actbio.2015.07.039. Epub 2015 Jul 27. Acta Biomater. 2015. PMID: 26225735 Free PMC article.
-
Evaluation of hemocompatibility and endothelialization of hybrid poly(vinyl alcohol) (PVA)/gelatin polymer films.J Biomed Mater Res B Appl Biomater. 2013 Nov;101(8):1549-59. doi: 10.1002/jbm.b.32977. Epub 2013 Jul 11. J Biomed Mater Res B Appl Biomater. 2013. PMID: 23846987
-
Fucoidan and topography modification improved in situ endothelialization on acellular synthetic vascular grafts.Bioact Mater. 2022 Oct 27;22:535-550. doi: 10.1016/j.bioactmat.2022.10.011. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36330164 Free PMC article.
-
Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: a review.Biomater Sci. 2016 Dec 20;5(1):22-37. doi: 10.1039/c6bm00618c. Biomater Sci. 2016. PMID: 27942617 Review.
-
Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications.Chem Soc Rev. 2015 Aug 7;44(15):5680-742. doi: 10.1039/c4cs00483c. Epub 2015 May 29. Chem Soc Rev. 2015. PMID: 26023741 Review.
Cited by
-
Changing compliance of poly(vinyl alcohol) tubular scaffold for vascular graft applications through modifying interlayer adhesion and crosslinking density.Front Mater. 2021 Jan;7:595295. doi: 10.3389/fmats.2020.595295. Epub 2021 Jan 14. Front Mater. 2021. PMID: 36936730 Free PMC article.
-
The Structural Characteristics of Seaweed Polysaccharides and Their Application in Gel Drug Delivery Systems.Mar Drugs. 2020 Dec 21;18(12):658. doi: 10.3390/md18120658. Mar Drugs. 2020. PMID: 33371266 Free PMC article. Review.
-
Exploring marine glycans: structure, function, and the frontier of chemical synthesis.RSC Chem Biol. 2025 Jun 4;6(8):1195-1213. doi: 10.1039/d5cb00090d. eCollection 2025 Jul 30. RSC Chem Biol. 2025. PMID: 40534732 Free PMC article. Review.
-
Evaluation of the Effect of Crosslinking Method of Poly(Vinyl Alcohol) Hydrogels on Thrombogenicity.Cardiovasc Eng Technol. 2020 Aug;11(4):448-455. doi: 10.1007/s13239-020-00474-y. Epub 2020 Jun 30. Cardiovasc Eng Technol. 2020. PMID: 32607901 Free PMC article.
-
Hemocompatibility of micropatterned biomaterial surfaces is dependent on topographical feature size.Front Physiol. 2022 Sep 19;13:983187. doi: 10.3389/fphys.2022.983187. eCollection 2022. Front Physiol. 2022. PMID: 36200053 Free PMC article.
References
-
- Bos GW, Poot AA, Beugeling T, van Aken WG, Feijen J, Small-Diameter Vascular Graft Prostheses: Current Status, Archives of Physiology and Biochemistry 106(2) (1998) 100–115. - PubMed
-
- Bergan JJ, Veith FJ, Bernhard VM, Yao JST, Flinn WR, Gupta SK, Scher LA, Samson RH, Towne JB, Randomization of autogenous vein and polytetrafluoroethylene grafts in femoral-distal reconstruction, Surgery 92(6) (1982) 921–930. - PubMed
-
- Pevec WC, Darling RC, L’Italien GI, Abbott WM, Femoropopliteal reconstruction with knitted, nonvelour Dacron versus expanded polytetrafluoroethylene, Journal of Vascular Surgery 16(1) (1992) 60–65. - PubMed
-
- Zilla P, Bezuidenhout D, Human P, Prosthetic vascular grafts: Wrong models, wrong questions and no healing, Biomaterials 28(34) (2007) 5009–5027. - PubMed
-
- Hiob MA, She S, Muiznieks LD, Weiss AS, Biomaterials and Modifications in the Development of Small-Diameter Vascular Grafts, ACS Biomaterials Science & Engineering 3(5) (2016) 712–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

