Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders
- PMID: 32305005
- PMCID: PMC7162980
- DOI: 10.1016/j.redox.2020.101535
Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders
Erratum in
-
Corrigendum to "Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders" [Redox Biology 32, 2020, 101535].Redox Biol. 2021 Aug;44:101955. doi: 10.1016/j.redox.2021.101955. Epub 2021 Mar 31. Redox Biol. 2021. PMID: 33811001 Free PMC article. No abstract available.
Abstract
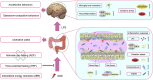
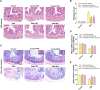
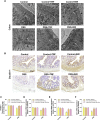
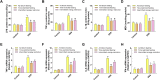
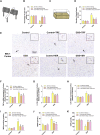
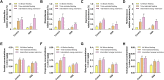
Intermittent fasting (IF) has been reported to have beneficial effects on improving gut function via lowering gut inflammation and altering the gut microbiome diversity. In this study, we aimed to investigate the differential effects of three different common IF treatments, alternate day fasting (ADF), time-restricted fasting (TRF), and intermittent energy restriction (IER), on a dextran sodium sulfate (DSS)-induced colitis mouse model. The results indicated that TRF and IER, but not ADF improved the survival rates of the colitis mice. TRF and IER, but not ADF, reversed the colitis pathological development by improving the gut barrier integrity and colon length. Importantly, TRF and IER suppressed the inflammatory responses and oxidative stress in colon tissues. Interestingly, TRF and IER also attenuated colitis-related anxiety-like and obsessive-compulsive disorder behavior and alleviated the neuroinflammation and oxidative stress. TRF and IER also altered the gut microbiota composition, including the decrease of the enrichments of colitis-related microbes such as Shigella and Escherichia Coli, and increase of the enrichments of anti-inflammatory-related microbes. TRF and IER also improved the short chain fatty acid formation in colitis mice. In conclusion, the TRF and IER but not ADF exhibited the protective effects against colitis and related behavioral disorders, which could be partly explained by improving the gut microbiome compositions and preventing gut leak, and consequently suppressing the inflammation and oxidative damages in both colon and brain. The current research indicates that proper IF regimens could be effective strategies for nutritional intervention for the prevention and treatment of colitis.
Keywords: Anxiety-like behavior; Colitis; Gut microbes; Inflammation; Intermittent fasting; Oxidative stress.
Copyright © 2020. Published by Elsevier B.V.
Figures


Similar articles
-
The antioxidant strain Lactiplantibacillus plantarum AS21 and Clostridium butyricum ameliorate DSS-induced colitis in mice by remodeling the assembly of intestinal microbiota and improving gut functions.Food Funct. 2024 Feb 19;15(4):2022-2037. doi: 10.1039/d3fo05337g. Food Funct. 2024. PMID: 38289370
-
Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut-Brain Axis Balance.J Agric Food Chem. 2020 Apr 1;68(13):3963-3975. doi: 10.1021/acs.jafc.0c00196. Epub 2020 Mar 23. J Agric Food Chem. 2020. PMID: 32162923
-
Oral Supplementation of Tocotrienol-Rich Fraction Alleviates Severity of Ulcerative Colitis in Mice.J Nutr Sci Vitaminol (Tokyo). 2019;65(4):318-327. doi: 10.3177/jnsv.65.318. J Nutr Sci Vitaminol (Tokyo). 2019. PMID: 31474681
-
Effect of Intermittent Fasting on Immune Parameters and Intestinal Inflammation.Nutrients. 2024 Nov 19;16(22):3956. doi: 10.3390/nu16223956. Nutrients. 2024. PMID: 39599741 Free PMC article. Review.
-
Effects of intermittent fasting on glucose and lipid metabolism.Proc Nutr Soc. 2017 Aug;76(3):361-368. doi: 10.1017/S0029665116002986. Epub 2017 Jan 16. Proc Nutr Soc. 2017. PMID: 28091348 Review.
Cited by
-
Time-restricted feeding attenuated hypertension-induced cardiac remodeling by modulating autophagy levels in spontaneously hypertensive rats.Sci Rep. 2025 May 15;15(1):16973. doi: 10.1038/s41598-025-01587-x. Sci Rep. 2025. PMID: 40374761 Free PMC article.
-
Dietary Risk Factors and Eating Behaviors in Peripheral Arterial Disease (PAD).Int J Mol Sci. 2022 Sep 16;23(18):10814. doi: 10.3390/ijms231810814. Int J Mol Sci. 2022. PMID: 36142725 Free PMC article. Review.
-
Treadmill Exercise Reverses the Adverse Effects of Intermittent Fasting on Behavior and Cortical Spreading Depression in Young Rats.Brain Sci. 2023 Dec 17;13(12):1726. doi: 10.3390/brainsci13121726. Brain Sci. 2023. PMID: 38137174 Free PMC article.
-
Studying the Relationship of Intermittent Fasting and β-Amyloid in Animal Model of Alzheimer's Disease: A Scoping Review.Nutrients. 2020 Oct 21;12(10):3215. doi: 10.3390/nu12103215. Nutrients. 2020. PMID: 33096730 Free PMC article.
-
Fasting: From Physiology to Pathology.Adv Sci (Weinh). 2023 Mar;10(9):e2204487. doi: 10.1002/advs.202204487. Epub 2023 Feb 3. Adv Sci (Weinh). 2023. PMID: 36737846 Free PMC article. Review.
References
-
- Adams S.M., Bornemann P.H. Ulcerative colitis. Am. Fam. Physician. 2013;87:699–705. - PubMed
-
- Navabi S., Gorrepati V.S., Yadav S., Chintanaboina J., Maher S., Demuth P., Stern B., Stuart A., Tinsley A., Clarke K., Williams E.D., Coates M.D. Influences and impact of anxiety and depression in the setting of inflammatory bowel disease. Inflamm. Bowel Dis. 2018;24:2303–2308. - PubMed
-
- Neuendorf R., Harding A., Stello N., Hanes D., Wahbeh H. Depression and anxiety in patients with Inflammatory Bowel Disease: a systematic review. J. Psychosom. Res. 2016;87:70–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

