Can post-exposure prophylaxis for COVID-19 be considered as an outbreak response strategy in long-term care hospitals?
- PMID: 32305587
- PMCID: PMC7162746
- DOI: 10.1016/j.ijantimicag.2020.105988
Can post-exposure prophylaxis for COVID-19 be considered as an outbreak response strategy in long-term care hospitals?
Abstract
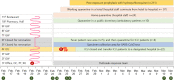
In the context of the ongoing global outbreak of coronavirus disease 2019 (COVID-19), management of exposure events is a concern. Long-term care hospitals (LTCHs) are particularly vulnerable to cluster outbreaks because facilities for patient isolation and healthcare personnel to care for these patients in isolation are difficult to arrange in a large outbreak situation. Although several drugs have been proposed as treatment options, there are no data on the effectiveness and safety of post-exposure prophylaxis (PEP) for COVID-19. After a large COVID-19 exposure event in an LTCH in Korea, PEP using hydroxychloroquine (HCQ) was administered to 211 individuals, including 189 patients and 22 careworkers, whose baseline polymerase chain reaction (PCR) tests for COVID-19 were negative. PEP was completed in 184 (97.4%) patients and 21 (95.5%) careworkers without serious adverse events. At the end of 14 days of quarantine, all follow-up PCR tests were negative. Based on our experience, further clinical studies are recommended for COVID-19 PEP.
Keywords: COVID-19; Hydroxychloroquine; Long-term care hospital; Post-exposure prophylaxis; SARS-CoV-2.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- Korean Society of Infectious Diseases KSoPID, Korean Society of Epidemiology, Korean Society for Antimicrobial Therapy, Korean Society for Healthcare-associated Infection Control and Prevention, and Korea Centers for Disease Control and Prevention Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. - PMC - PubMed
-
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquinone for the treatment of severe acute respiratory syndrome coronarvirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. ciaa237. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

