Zika virus envelope nanoparticle antibodies protect mice without risk of disease enhancement
- PMID: 32305868
- PMCID: PMC7186774
- DOI: 10.1016/j.ebiom.2020.102738
Zika virus envelope nanoparticle antibodies protect mice without risk of disease enhancement
Abstract
Background: Zika virus (ZIKV), an arbovirus capable of causing neurological abnormalities, is a recognised human pathogen, for which a vaccine is required. As ZIKV antibodies can mediate antibody-dependent enhancement (ADE) of dengue virus (DENV) infection, a ZIKV vaccine must not only protect against ZIKV but must also not sensitise vaccinees to severe dengue.
Methods: The N-terminal 80% of ZIKV envelope protein (80E) was expressed in Pichia pastoris and its capacity to self-assemble into particulate structures evaluated using dynamic light scattering and electron microscopy. Antigenic integrity of the 80E protein was evaluated using ZIKV-specific monoclonal antibodies. Its immunogenicity and protective efficacy were assessed in BALB/c and C57BL/6 Stat2-/- mice, respectively. Its capacity to enhance DENV and ZIKV infection was assessed in AG129 and C57BL/6 Stat2-/- mice, respectively.
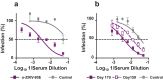
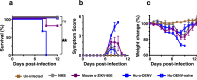
Findings: ZIKV-80E protein self-assembled into discrete nanoparticles (NPs), which preserved the antigenic integrity of neutralising epitopes on E domain III (EDIII) and elicited potent ZIKV-neutralising antibodies predominantly against this domain in BALB/c mice. These antibodies conferred statistically significant protection in vivo (p = 0.01, Mantel-Cox test), and did not exacerbate sub-lethal DENV-2 or ZIKV challenges in vivo.
Interpretation: Yeast-expressed ZIKV-80E, which forms highly immunogenic EDIII-displaying NPs, elicits ZIKV EDIII-specific antibodies capable of offering significant protection in vivo, without the potential risk of ADE upon subsequent DENV-2 or ZIKV infection. This offers a promising vaccine candidate for further development.
Funding: This study was supported partly by ICGEB, India, and by NIAID, USA.
Keywords: AG129; Antibody-dependent enhancement; C57BL/6 Stat2(−/−); Nanoparticles; Pichia pastoris;Dengue virus; VLPs; Zika virus vaccine.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Nothing to disclose: GB, JAA; Grant from University Grants Commission, RKS; Grant from ICGEB: RS, VR, SS, NK; Grant from NIAID: JKL, FK; Patent 201911014359 pending: RS, RKS, VR, UA, SS, NK.
Figures






References
-
- Pierson T.C., Diamond M.S. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields virology. 6 ed. Wolters Kluwer and Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 747–794.
-
- Pierson T.C., Diamond M.S. The emergence of Zika virus and its new clinical syndromes. Nature. 2018;560:573–581. - PubMed
-
- Gatherer D., Kohl A. Zika virus: a previously slow pandemic spreads rapidly through the Americas. J Gen Virol. 2016;97:269–273. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

