Radiosurgery for ventricular tachycardia: preclinical and clinical evidence and study design for a German multi-center multi-platform feasibility trial (RAVENTA)
- PMID: 32306083
- PMCID: PMC7588361
- DOI: 10.1007/s00392-020-01650-9
Radiosurgery for ventricular tachycardia: preclinical and clinical evidence and study design for a German multi-center multi-platform feasibility trial (RAVENTA)
Abstract
Background: Single-session high-dose stereotactic radiotherapy (radiosurgery) is a new treatment option for otherwise untreatable patients suffering from refractory ventricular tachycardia (VT). In the initial single-center case studies and feasibility trials, cardiac radiosurgery has led to significant reductions of VT burden with limited toxicities. However, the full safety profile remains largely unknown.
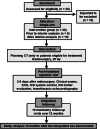
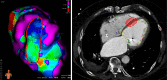
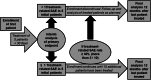
Methods/design: In this multi-center, multi-platform clinical feasibility trial which we plan is to assess the initial safety profile of radiosurgery for ventricular tachycardia (RAVENTA). High-precision image-guided single-session radiosurgery with 25 Gy will be delivered to the VT substrate determined by high-definition endocardial electrophysiological mapping. The primary endpoint is safety in terms of successful dose delivery without severe treatment-related side effects in the first 30 days after radiosurgery. Secondary endpoints are the assessment of VT burden, reduction of implantable cardioverter defibrillator (ICD) interventions [shock, anti-tachycardia pacing (ATP)], mid-term side effects and quality-of-life (QoL) in the first year after radiosurgery. The planned sample size is 20 patients with the goal of demonstrating safety and feasibility of cardiac radiosurgery in ≥ 70% of the patients. Quality assurance is provided by initial contouring and planning benchmark studies, joint multi-center treatment decisions, sequential patient safety evaluations, interim analyses, independent monitoring, and a dedicated data and safety monitoring board.
Discussion: RAVENTA will be the first study to provide the initial robust multi-center multi-platform prospective data on the therapeutic value of cardiac radiosurgery for ventricular tachycardia.
Trial registration number: NCT03867747 (clinicaltrials.gov). Registered March 8, 2019. The study was initiated on November 18th, 2019, and is currently recruiting patients.
Keywords: Cardiac arrhythmia; Clinical feasibility trial; Multi-center; Multi-platform; Radioablation; Radiosurgery; SBRT; Stereotactic body radiotherapy; Ventricular tachycardia.
Conflict of interest statement
OB reports to have been an employee of CyberHeart Inc. (Sunnyvale, CA, USA) from 2008 to 2010 during the initial preclinical studies for cardiac radiosurgery, though he reports no financial ties or obligations or conflict of interest to or with the company or its legal predecessor Varian Inc. (Sunnyvale, CA, USA). DB reports personal fees from Siemens AG, personal fees from NB Capital Research GmbH, personal fees from NB Capital ApS, personal fees from b.e. Imaging GmbH, outside the submitted work. DK has received honoraria from Merck Sharp & Dome, outside of the submitted work. BR received travel grants and personal fees from Boston Scientific Medizintechnik GmbH, outside of the submitted work. All other authors report no competing interests.
Figures
References
-
- Shivkumar K. Catheter ablation of ventricular arrhythmias. N Engl J Med. 2019;380:1555–1564. - PubMed
-
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15(10):e190–e252. - PubMed
-
- Dukkipati SR, Koruth JS, Choudry S, et al. Catheter ablation of ventricular tachycardia in structural heart disease: indications, strategies, and outcomes—part II. J Am Coll Cardiol. 2017;70(23):2924–2941. - PubMed
-
- Sapp JL, Wells GA, Parkash R, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375(2):111–121. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

