Safety and Efficacy of Exenatide Once Weekly in Participants with Type 2 Diabetes and Stage 2/3 Chronic Kidney Disease
- PMID: 32306296
- PMCID: PMC7324446
- DOI: 10.1007/s13300-020-00815-z
Safety and Efficacy of Exenatide Once Weekly in Participants with Type 2 Diabetes and Stage 2/3 Chronic Kidney Disease
Erratum in
-
Correction to: Safety and Efficacy of Exenatide Once Weekly in Participants with Type 2 Diabetes and Stage 2/3 Chronic Kidney Disease.Diabetes Ther. 2020 Dec;11(12):3011-3013. doi: 10.1007/s13300-020-00842-w. Diabetes Ther. 2020. PMID: 33011938 Free PMC article.
Abstract
Introduction: The safety and efficacy of exenatide once weekly (EQW) is overall well established. EQW is primarily renally eliminated. In this study, the efficacy and renal and gastrointestinal tolerability of EQW were summarised in participants with type 2 diabetes and chronic kidney disease stage 3 (CKD3; moderate renal impairment; estimated glomerular filtration rate [eGFR] ≥ 30 to < 60 mL/min/1.73 m2) or CKD stage 2 (CKD2; mild renal impairment; eGFR ≥ 60 to < 90 mL/min/1.73 m2).
Methods: Data on participants with type 2 diabetes and baseline CKD3 or CKD2 from eight phase 3, double-blind or open-label studies with 26- or 28-week controlled treatment periods were pooled. Participants received EQW or a placebo/non-glucagon-like peptide-1 receptor agonist comparator (sitagliptin, metformin, pioglitazone, dapagliflozin and insulin).
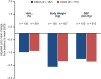
Results: Participants with baseline CKD3 (N = 182) or CKD2 (N = 772) receiving EQW differed in a number of baseline characteristics, such as age < 65 years, race, mean body mass index and mean type 2 diabetes duration, whereas mean blood pressure and glycated haemoglobin (HbA1c) were similar. Mean reductions in HbA1c, body weight and systolic blood pressure from baseline to week 26/28 in participants receiving EQW were similar between the CKD subgroups. The proportions of participants (CKD3 and CKD2) with any adverse event (AE) were 81% and 72%, respectively, for EQW and 74% and 68%, respectively, for all comparators; those for serious AEs were 2.7% and 3.4%, respectively, for EQW and 6% and 5%, respectively, for all comparators. Gastrointestinal AE rates were higher in the EQW CKD3 subgroup (42.2% of participants) than in the CKD2 (32.8%) subgroup, although rates for nausea and vomiting were similar. There were no dehydration events; one participant in each treatment group had a serious AE of acute kidney injury (EQW with CKD3, n = 1; pioglitazone with CKD2, n = 1).
Conclusion: Exenatide once weekly was well tolerated and demonstrated similar efficacy in participants with type 2 diabetes with mild and moderate renal impairment.
Trial registration: ClinicalTrials.gov identifiers: NCT00637273, NCT00676338, NCT02229383, NCT02229396, NCT00641056, NCT01652729, NCT00935532, NCT01003184.
Keywords: CKD2; CKD3; Chronic renal insufficiency; Exenatide; Glucagon-like peptide 1; Pooled analysis; Safety; Type 2 diabetes mellitus.
Figures
References
-
- European Medicines Agency. Bydureon. Summary of product characteristics. Södertälje, Sweden: AstraZeneca AB; 2019. https://www.ema.europa.eu/en/documents/product-information/bydureon-epar.... Accessed 22 Jan 2020.
-
- Federal Drug Administration. Bydureon® BCise™ prescribing information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209210s000lbl.pdf. Accessed 22 Jan 2020.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

