Nuchal translucency of 3.0-3.4 mm an indication for NIPT or microarray? Cohort analysis and literature review
- PMID: 32306377
- PMCID: PMC7318216
- DOI: 10.1111/aogs.13877
Nuchal translucency of 3.0-3.4 mm an indication for NIPT or microarray? Cohort analysis and literature review
Abstract
Introduction: Currently fetal nuchal translucency (NT) ≥3.5 mm is an indication for invasive testing often followed by chromosomal microarray. The aim of this study was to assess the risks for chromosomal aberrations in fetuses with an NT 3.0-3.4 mm, to determine whether invasive prenatal testing would be relevant in these cases and to assess the residual risks in fetuses with normal non-invasive prenatal test (NIPT) results.
Material and methods: A retrospective study and meta-analysis of literature cases with NT between 3.0 and 3.4 mm and 2 cohorts of pregnant women referred for invasive testing and chromosomal microarray was performed: Rotterdam region (with a risk >1:200 and NT between 3.0 and 3.4 mm) tested in the period July 2012 to June 2019 and Central Denmark region (with a risk >1:300 and NT between 3.0 and 3.4 mm) tested between September 2015 and December 2018.
Results: A total of 522 fetuses were referred for invasive testing and chromosomal microarray. Meta-analysis indicated that in 1:7.4 (13.5% [95% CI 8.2%-21.5%]) fetuses a chromosomal aberration was diagnosed. Of these aberrant cases, 47/68 (69%) involved trisomy 21, 18, and 13 and would potentially be detected by all NIPT approaches. The residual risk for missing a (sub)microscopic chromosome aberration depends on the NIPT approach and is highest if NIPT was performed only for common trisomies-1:21 (4.8% [95% CI 3.2%-7.3%]). However, it may be substantially lowered if a genome-wide 10-Mb resolution NIPT test was offered (~1:464).
Conclusions: Based on these data, we suggest that the NT cut-off for invasive testing could be 3.0 mm (instead of 3.5 mm) because of the high risk of 1:7.4 for a chromosomal aberration. If women were offered NIPT first, there would be a significant diagnostic delay because all abnormal NIPT results need to be confirmed by diagnostic testing. If the woman had already received a normal NIPT result, the residual risk of 1:21 to 1:464 for chromosome aberrations other than common trisomies, dependent on the NIPT approach, should be raised. If a pregnant woman declines invasive testing, but still wants a test with a broader coverage of clinically significant conditions then the genome-wide >10-Mb resolution NIPT test, which detects most aberrations, could be proposed.
Keywords: microarray; microdeletion; non-invasive prenatal test; nuchal translucency; prenatal diagnosis; submicroscopic chromosomal abnormalities.
© 2020 The Authors. Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic.
Conflict of interest statement
None.
Figures
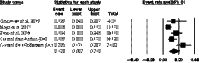
References
-
- Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal‐translucency thickness at 10–14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet. 1998;352(9125):343‐346. - PubMed
-
- Souka AP, Von Kaisenberg CS, Hyett JA, Sonek JD, Nicolaides KH. Increased nuchal translucency with normal karyotype. Am J Obstet Gynecol. 2005;192(4):1005‐1021. - PubMed
-
- Bilardo CM, Muller MA, Pajkrt E, Clur SA, van Zalen MM, Bijlsma EK. Increased nuchal translucency thickness and normal karyotype: time for parental reassurance. Ultrasound Obstet Gynecol. 2007;30(1):11‐18. - PubMed
-
- Lund IC, Christensen R, Petersen OB, Vogel I, Vestergaard EM. Chromosomal microarray in fetuses with increased nuchal translucency. Ultrasound Obstet Gynecol. 2015;45(1):95‐100. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

