Interleukin-33 alleviates psoriatic inflammation by suppressing the T helper type 17 immune response
- PMID: 32306382
- PMCID: PMC7370137
- DOI: 10.1111/imm.13203
Interleukin-33 alleviates psoriatic inflammation by suppressing the T helper type 17 immune response
Abstract
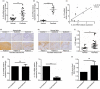
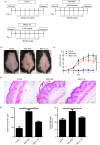
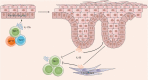
Psoriasis is a chronic inflammatory skin disease with unclear pathogenesis. Interleukin-33 (IL-33) is highly expressed in patients with psoriasis, but its role in psoriasis is unknown. The aim of this study was to investigate the possible role of IL-33 in the pathogenesis and treatment of psoriasis. IL-33 expression was determined using enzyme-linked immunosorbent assay, real-time fluorescent quantitative polymerase chain reaction and immunohistochemical staining. CD4+ T cells were sorted using magnetic beads and treated with or without IL-33. Imiquimod (IMQ) was used to induce psoriatic inflammation in mice. The frequency of immune cells was determined using flow cytometry. The cytokine level in mouse skin was measured using cytometric bead array. Our results showed that IL-33 was highly expressed in the lesional skin and serum of patients with moderate-to-severe plaque psoriasis. IL-33 inhibited the expression of IL-17 in CD4+ T cells of psoriasis patients. Subcutaneous injection of IL-33 alleviated the IMQ-induced psoriatic inflammation in mice, reduced tumor necrosis factor-α and IL-23 expression, and decreased the proportion of T helper type 17 (Th17) cells in the skin-draining lymph nodes in the mice. Our results suggest that IL-33 plays a protective role in the pathogenesis of psoriasis by suppressing Th17 cell differentiation and function. The potential therapeutic effect of IL-33 in treating psoriasis warrants further investigation.
Keywords: T helper type 17 cells; interleukin-17; interleukin-33; psoriasis.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures


Similar articles
-
Decreased expression of IL-27 in moderate-to-severe psoriasis and its anti-inflammation role in imiquimod-induced psoriasis-like mouse model.J Dermatol Sci. 2017 Feb;85(2):115-123. doi: 10.1016/j.jdermsci.2016.11.011. Epub 2016 Nov 28. J Dermatol Sci. 2017. PMID: 27939414
-
Effect of γ-secretase inhibitor on Th17 cell differentiation and function of mouse psoriasis-like skin inflammation.J Transl Med. 2018 Mar 10;16(1):59. doi: 10.1186/s12967-018-1442-6. J Transl Med. 2018. PMID: 29523162 Free PMC article.
-
Indirubin ameliorates imiquimod-induced psoriasis-like skin lesions in mice by inhibiting inflammatory responses mediated by IL-17A-producing γδ T cells.Mol Immunol. 2018 Sep;101:386-395. doi: 10.1016/j.molimm.2018.07.011. Epub 2018 Jul 29. Mol Immunol. 2018. PMID: 30064075
-
Targeting interleukin-22 in psoriasis.Inflammation. 2014 Feb;37(1):94-9. doi: 10.1007/s10753-013-9715-y. Inflammation. 2014. PMID: 23978911 Review.
-
Interleukin 17A: toward a new understanding of psoriasis pathogenesis.J Am Acad Dermatol. 2014 Jul;71(1):141-50. doi: 10.1016/j.jaad.2013.12.036. Epub 2014 Mar 18. J Am Acad Dermatol. 2014. PMID: 24655820 Review.
Cited by
-
Decoding IL-23 Signaling Cascade for New Therapeutic Opportunities.Cells. 2020 Sep 7;9(9):2044. doi: 10.3390/cells9092044. Cells. 2020. PMID: 32906785 Free PMC article. Review.
-
Paradoxical pustular psoriasis induced by tumor necrosis factor inhibitor with elevated interferon-alpha in an ankylosing spondylitis patient: A case report.JAAD Case Rep. 2025 Jun 9;62:65-68. doi: 10.1016/j.jdcr.2025.05.012. eCollection 2025 Aug. JAAD Case Rep. 2025. PMID: 40678000 Free PMC article. No abstract available.
-
Activity-attenuated serum albumin-fused interleukin-33 suppresses experimental autoimmune encephalomyelitis.Cell Rep Med. 2025 Jul 15;6(7):102231. doi: 10.1016/j.xcrm.2025.102231. Epub 2025 Jul 7. Cell Rep Med. 2025. PMID: 40628263 Free PMC article.
-
Serum Metabolomic Profiling Reveals the Amelioration Effect of Methotrexate on Imiquimod-Induced Psoriasis in Mouse.Front Pharmacol. 2020 Nov 19;11:558629. doi: 10.3389/fphar.2020.558629. eCollection 2020. Front Pharmacol. 2020. PMID: 33364938 Free PMC article.
-
Cross-sectional study of proteomic differences between moderate and severe psoriasis.Sci Rep. 2025 Jan 27;15(1):3387. doi: 10.1038/s41598-025-87252-9. Sci Rep. 2025. PMID: 39870771 Free PMC article.
References
-
- Srivastava A, Nikamo P, Lohcharoenkal W, Li D, Meisgen F, Xu Landen N et al MicroRNA‐146a suppresses IL‐17‐mediated skin inflammation and is genetically associated with psoriasis. J Allergy Clin Immunol 2017; 139:550–61. - PubMed
-
- Ding X, Wang T, Shen Y, Wang X, Zhou C, Tian S et al Prevalence of psoriasis in China: a population‐based study in six cities. Euro J Dermatol 2012; 22:663–7. - PubMed
-
- Campanati A, Molinelli E, Brisigotti V, Offidani A. Biologic therapy in psoriasis (Part I): efficacy and safety of tumor necrosis factor‐α inhibitors. Curr Pharm Biotechnol 2017; 18:945–63. - PubMed
-
- Molinelli E, Campanati A, Brisigotti V, Offidani A. Biologic therapy in psoriasis (Part II): efficacy and safety of new treatment targeting IL23/IL‐17 pathways. Curr Pharm Biotechnol 2017; 18:964–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

