ALS-associated genes in SCA2 mouse spinal cord transcriptomes
- PMID: 32307524
- PMCID: PMC7322574
- DOI: 10.1093/hmg/ddaa072
ALS-associated genes in SCA2 mouse spinal cord transcriptomes
Abstract
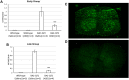
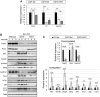
The spinocerebellar ataxia type 2 (SCA2) gene ATXN2 has a prominent role in the pathogenesis and treatment of amyotrophic lateral sclerosis (ALS). In addition to cerebellar ataxia, motor neuron disease is often seen in SCA2, and ATXN2 CAG repeat expansions in the long normal range increase ALS risk. Also, lowering ATXN2 expression in TDP-43 ALS mice prolongs their survival. Here we investigated the ATXN2 relationship with motor neuron dysfunction in vivo by comparing spinal cord (SC) transcriptomes reported from TDP-43 and SOD1 ALS mice and ALS patients with those from SCA2 mice. SC transcriptomes were determined using an SCA2 bacterial artificial chromosome mouse model expressing polyglutamine expanded ATXN2. SCA2 cerebellar transcriptomes were also determined, and we also investigated the modification of gene expression following treatment of SCA2 mice with an antisense oligonucleotide (ASO) lowering ATXN2 expression. Differentially expressed genes (DEGs) defined three interconnected pathways (innate immunity, fatty acid biosynthesis and cholesterol biosynthesis) in separate modules identified by weighted gene co-expression network analysis. Other key pathways included the complement system and lysosome/phagosome pathways. Of all DEGs in SC, 12.6% were also dysregulated in the cerebellum. Treatment of mice with an ATXN2 ASO also modified innate immunity, the complement system and lysosome/phagosome pathways. This study provides new insights into the underlying molecular basis of SCA2 SC phenotypes and demonstrates annotated pathways shared with TDP-43 and SOD1 ALS mice and ALS patients. It also emphasizes the importance of ATXN2 in motor neuron degeneration and confirms ATXN2 as a therapeutic target.
© The Author(s) 2020. Published by Oxford University Press.
Figures



Similar articles
-
ATXN2-AS, a gene antisense to ATXN2, is associated with spinocerebellar ataxia type 2 and amyotrophic lateral sclerosis.Ann Neurol. 2016 Oct;80(4):600-15. doi: 10.1002/ana.24761. Ann Neurol. 2016. PMID: 27531668 Free PMC article.
-
Distinct patterns of cerebral and spinal pathology along the spectrum of ATXN2-related disorders.J Neurol. 2025 Apr 9;272(5):330. doi: 10.1007/s00415-025-13037-9. J Neurol. 2025. PMID: 40204975
-
A quantitative high-throughput screen identifies compounds that lower expression of the SCA2-and ALS-associated gene ATXN2.J Biol Chem. 2022 Aug;298(8):102228. doi: 10.1016/j.jbc.2022.102228. Epub 2022 Jul 2. J Biol Chem. 2022. PMID: 35787375 Free PMC article.
-
Oligonucleotide therapeutics in neurodegenerative diseases.RNA Biol. 2018;15(6):707-714. doi: 10.1080/15476286.2018.1454812. Epub 2018 Jun 1. RNA Biol. 2018. PMID: 29560813 Free PMC article. Review.
-
The polyglutamine protein ATXN2: from its molecular functions to its involvement in disease.Cell Death Dis. 2024 Jun 14;15(6):415. doi: 10.1038/s41419-024-06812-5. Cell Death Dis. 2024. PMID: 38877004 Free PMC article. Review.
Cited by
-
Ataxin-2 gene: a powerful modulator of neurological disorders.Curr Opin Neurol. 2021 Aug 1;34(4):578-588. doi: 10.1097/WCO.0000000000000959. Curr Opin Neurol. 2021. PMID: 34010218 Free PMC article. Review.
-
TDP-43 and Inflammation: Implications for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia.Int J Mol Sci. 2021 Jul 21;22(15):7781. doi: 10.3390/ijms22157781. Int J Mol Sci. 2021. PMID: 34360544 Free PMC article. Review.
-
Control of innate immunity and lipid biosynthesis in neurodegeneration.Front Mol Neurosci. 2024 Jul 25;17:1402055. doi: 10.3389/fnmol.2024.1402055. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39156128 Free PMC article. Review.
-
ATAXIN-2 intermediate-length polyglutamine expansions elicit ALS-associated metabolic and immune phenotypes.Nat Commun. 2024 Aug 29;15(1):7484. doi: 10.1038/s41467-024-51676-0. Nat Commun. 2024. PMID: 39209824 Free PMC article.
-
Consensus Paper: Strengths and Weaknesses of Animal Models of Spinocerebellar Ataxias and Their Clinical Implications.Cerebellum. 2022 Jun;21(3):452-481. doi: 10.1007/s12311-021-01311-1. Epub 2021 Aug 10. Cerebellum. 2022. PMID: 34378174 Free PMC article.
References
-
- Pulst S.M., Nechiporuk A., Nechiporuk T., Gispert S., Chen X.N., Lopes-Cendes I., Pearlman S., Starkman S., Orozco-Diaz G., Lunkes A. et al. (1996) Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat. Genet., 14, 269–276. - PubMed
-
- Fernandez M., McClain M.E., Martinez R.A., Snow K., Lipe H., Ravits J., Bird T.D. and La Spada A.R. (2000) Late-onset SCA2: 33 CAG repeats are sufficient to cause disease. Neurology, 55, 569–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

