Central afferents to the nucleus of the solitary tract in rats and mice
- PMID: 32307700
- PMCID: PMC7942812
- DOI: 10.1002/cne.24927
Central afferents to the nucleus of the solitary tract in rats and mice
Abstract
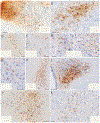
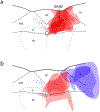
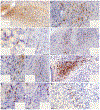
The nucleus of the solitary tract (NTS) regulates life-sustaining functions ranging from appetite and digestion to heart rate and breathing. It is also the brain's primary sensory nucleus for visceral sensations relevant to symptoms in medical and psychiatric disorders. To better understand which neurons may exert top-down control over the NTS, here we provide a brain-wide map of all neurons that project axons directly to the caudal, viscerosensory NTS, focusing on a medial subregion with aldosterone-sensitive HSD2 neurons. Injecting an axonal tracer (cholera toxin b) into the NTS produces a similar pattern of retrograde labeling in rats and mice. The paraventricular hypothalamic nucleus (PVH), lateral hypothalamic area, and central nucleus of the amygdala (CeA) contain the densest concentrations of NTS-projecting neurons. PVH afferents are glutamatergic (express Slc17a6/Vglut2) and are distinct from neuroendocrine PVH neurons. CeA afferents are GABAergic (express Slc32a1/Vgat) and are distributed largely in the medial CeA subdivision. Other retrogradely labeled neurons are located in a variety of brain regions, including the cerebral cortex (insular and infralimbic areas), bed nucleus of the stria terminalis, periaqueductal gray, Barrington's nucleus, Kölliker-Fuse nucleus, hindbrain reticular formation, and rostral NTS. Similar patterns of retrograde labeling result from tracer injections into different NTS subdivisions, with dual retrograde tracing revealing that many afferent neurons project axon collaterals to both the lateral and medial NTS subdivisions. This information provides a roadmap for studying descending axonal projections that may influence visceromotor systems and visceral "mind-body" symptoms.
Keywords: hypothalamus; infralimbic; insular cortex; medial prefrontal cortex; nucleus of the solitary tract; nucleus tractus solitarii; nucleus tractus solitarius; paraventricular; retrograde tracing; solitary nucleus.
© 2020 Wiley Periodicals, Inc.
Figures









References
-
- Bailey TW, Hermes SM, Andresen MC, & Aicher SA (2006, November 15). Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci, 26(46), 11893–11902. 10.1523/JNEUROSCI.2044-06.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

