In Vivo Flow Cytometric Evaluation of Circulating Metastatic Pancreatic Tumor Cells after High-Intensity Focused Ultrasound Therapy
- PMID: 32307867
- PMCID: PMC7540359
- DOI: 10.1002/cyto.a.24014
In Vivo Flow Cytometric Evaluation of Circulating Metastatic Pancreatic Tumor Cells after High-Intensity Focused Ultrasound Therapy
Abstract
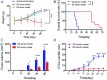
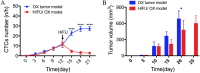
We examined our hypothesis that high-intensity focused ultrasound (HIFU) treatment of pancreatic ductal adenocarcinoma (PDAC) in nude mice models may lead to an increased occurrence of hematogenous metastasis. The human PDAC cell line BxPC-3 transfected with mCherry was implanted into nude mice to establish orthotopic and subcutaneous xenograft (OX and SX) tumor models. Mice were exposed to HIFU when tumor sizes reached approximately 200-300 mm3 . The OX and SX tumor models were monitored continuously for tumor growth characteristics and hematogenous metastasis using in vivo flow cytometric (IVFC) detection of circulating tumor cells (CTCs) from the pancreas. We chose an appropriate mouse model to further examine whether or not HIFU increases the potential risk of hematogenous metastasis, using IVFC detection. Our results showed that the CTC number was greater in the OX model than in the SX model. The CTC number in the OX model increased gradually over time, whereas the CTC number in the SX model remained low. Therefore, the OX model was better for studying tumor metastasis by IVFC detection. We found significantly decreased CTC numbers and tumor volume after HIFU ablation. Our results showed the applicability of the PDAC OX tumor model for studying the occurrence of tumor metastasis due to the generation of CTCs. HIFU ablation substantially restricted PDAC hematogenous metastasis and provided effective tumor control locally. © 2020 The Authors. Cytometry Part A published by Wiley Periodicals Inc., on behalf of International Society for Advancement of Cytometry.
Keywords: circulating tumor cells; high-intensity focused ultrasound; in vivo flow cytometry; pancreatic cancer.
© 2020 The Authors. Cytometry Part A published by Wiley Periodicals Inc., on behalf of International Society for Advancement of Cytometry.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

