Increased expression of microRNA-155-5p by alveolar type II cells contributes to development of lethal ARDS in H1N1 influenza A virus-infected mice
- PMID: 32308197
- PMCID: PMC7199462
- DOI: 10.1016/j.virol.2020.03.005
Increased expression of microRNA-155-5p by alveolar type II cells contributes to development of lethal ARDS in H1N1 influenza A virus-infected mice
Abstract
Alveolar type II (ATII) cells are essential to lung function and a primary site of influenza A virus (IAV) replication. Effects of IAV infection on ATII cell microRNA (miR) expression have not been comprehensively investigated. Infection of C57BL/6 mice with 10,000 or 100 pfu/mouse of IAV A/WSN/33 (H1N1) significantly altered expression of 73 out of 1908 mature murine miRs in ATII cells at 2 days post-infection (d.p.i.) and 253 miRs at 6 d.p.i. miR-155-5p (miR-155) showed the greatest increase in expression within ATII cells at both timepoints and the magnitude of this increase correlated with inoculum size and pulmonary edema severity. Influenza-induced lung injury was attenuated in C57BL/6-congenic miR-155-knockout mice without affecting viral replication. Attenuation of lung injury was dependent on deletion of miR-155 from stromal cells and was recapitulated in ATII cell-specific miR-155-knockout mice. These data suggest that ATII cell miR-155 is a potential therapeutic target for IAV-induced ARDS.
Keywords: Acute respiratory distress syndrome; Alveolar type II cell; Influenza A virus; Mouse; microRNA.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing interests.
Figures

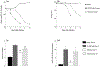

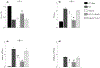



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

