Desflurane Preconditioning Protects Against Renal Ischemia-Reperfusion Injury and Inhibits Inflammation and Oxidative Stress in Rats Through Regulating the Nrf2-Keap1-ARE Signaling Pathway
- PMID: 32308368
- PMCID: PMC7138619
- DOI: 10.2147/DDDT.S223742
Desflurane Preconditioning Protects Against Renal Ischemia-Reperfusion Injury and Inhibits Inflammation and Oxidative Stress in Rats Through Regulating the Nrf2-Keap1-ARE Signaling Pathway
Abstract
Objective: Kidney is sensitive to ischemia-reperfusion (I/R) injury because of its special structure and function. In this study, we aimed to explore the mechanism of desflurane (DFE) preconditioning effecting on renal I/R injury in rats.
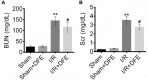
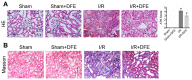
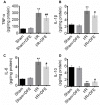
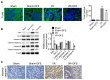
Methods: Renal I/R injury rats model was constructed, and the expressions of serum renal function parameters (blood urea nitrogen (BUN) and serum creatinine (SCr)) and lipid peroxidation-related factors were detected using corresponding commercial kits to assess the degrees of renal functional damage and oxidative stress. Hematoxylin--eosin (HE) staining and Masson trichrome staining were applied to measure the renal histologic damage. The expressions of inflammation-related factors were determined by ELISA assay. The cell apoptosis was analyzed using TUNEL, Western blot and immunohistochemistry (IHC). IHC was also used to detect the number of myeloperoxidase (MPO)-positive cells. The expressions of proteins associated with the Nrf2-Keap1-ARE pathway were assessed by Western blot and IHC.
Results: DFE preconditioning inhibited I/R injury-induced BUN and SCr increase and renal histologic injury in rats. Also, DFE suppressed the inflammation, apoptosis and oxidative stress caused by renal I/R injury in vivo. In addition, DFE preconditioning repressed peroxide-related factors (MDA, MPO and NO) expressions and promoted antioxidant-related factors (GSH, SOD, GPx and CAT) expressions. In addition, DFE promoted Nrf2-Keap1-ARE-related proteins including Nrf2, NQO1, HO-1, γ-GCS, GSR and GCLc expressions.
Conclusion: DFE preconditioning protected the kidney as well as inhibited the inflammation, cell apoptosis and oxidative stress in renal I/R injury rats by activating the Nrf2-Keap1-ARE signaling pathway.
Keywords: Nrf2-Keap1-ARE signaling pathway; animal model; desflurane; ischemia–reperfusion; kidney.
© 2020 Zheng et al.
Conflict of interest statement
Yan Zheng and Hui Lu are co-first authors. The authors declare that they have no competing interests in this work.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

