Soluble Biomarkers of Osteoporosis and Osteoarthritis, from Pathway Mapping to Clinical Trials: An Update
- PMID: 32308378
- PMCID: PMC7152733
- DOI: 10.2147/CIA.S242288
Soluble Biomarkers of Osteoporosis and Osteoarthritis, from Pathway Mapping to Clinical Trials: An Update
Abstract
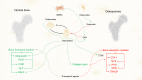
Serum biomarkers of osteoarticular diseases have been in the limelight of current clinical research trends. Laboratory validation of defined and candidate biomarkers for both osteoarthritis and osteoporosis is of key importance for future decisional algorithms in the diagnosis, monitoring, and prognosis of these diseases. The current guidelines recommend the use of collagen degradation remnants, eg, CTX-I and CTX-II, in the complementary diagnosis of both osteoporosis and osteoarthritis. Besides the collagen degradation markers, enzymes that regulate bone and articular metabolism are useful in the clinical evaluation of osteoarticular pathologies. Along these, several other recommended and new nominee molecules have been recently studied. Wnts and Wnt-related molecules have a cardinal role in the bone-joint homeostasis, making them a promising target not only for pharmaceutical modulation, but also to be considered as soluble biomarkers. Sclerostin and dickkopf, two inhibitor molecules of the Wnt/β-catenin signaling, might have a dual role in the assessment of the clinical manifestations of the osteoarticular unit. In osteoarthritis, besides fragments of collagen type II many pathway-related molecules have been studied and proposed for biomarker validation. The most serious limitation is that a significant proportion of studies lack statistical power due to the reduced number of cases enrolled. Serum biomarkers of bone and joint turnover markers represent an encouraging possibility for the diagnosis and prognosis of osteoarticular diseases, although further studies and laboratory validations should be carried out as to solely rely on them.
Keywords: biomarkers; osteoarthritis; osteoporosis.
© 2020 Nagy et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Group F-NBW. BEST (Biomarkers, Endpoints, and Other Tools) Resource; 2016. - PubMed
-
- Burch J, Rice S, Yang H, et al. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technol Assess (Rockv). 2014;18(11):1–180. doi: 10.3310/hta18110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

