Mannose Suppresses the Proliferation and Metastasis of Lung Cancer by Targeting the ERK/GSK-3β/β-Catenin/SNAIL Axis
- PMID: 32308412
- PMCID: PMC7135191
- DOI: 10.2147/OTT.S241816
Mannose Suppresses the Proliferation and Metastasis of Lung Cancer by Targeting the ERK/GSK-3β/β-Catenin/SNAIL Axis
Retraction in
-
Mannose Suppresses the Proliferation and Metastasis of Lung Cancer by Targeting the ERK/GSK-3β/β-Catenin/SNAIL Axis [Retraction].Onco Targets Ther. 2024 Jan 25;17:39-40. doi: 10.2147/OTT.S461193. eCollection 2024. Onco Targets Ther. 2024. PMID: 38292059 Free PMC article.
Abstract
Introduction: It has been found that mannose exerts antitumoural properties in vitro and in animal models. Whether mannose has potential anti-proliferative and anti-metastatic properties against non-small-cell lung cancer (NSCLC) is still unclear.
Methods: Here, we performed ex vivo experiments and established a nude mouse model to evaluate the anticancer effects of mannose on NSCLC cells and its effects on the ERK/GSK-3β/β-catenin/SNAIL axis. A CCK-8 assay was conducted to evaluate the effects of mannose on lung cancer cells (A549 and HCC827) and normal lung cells (HPAEpiC). Transwells were used to examine the motility of cancer cells. qRT-PCR was used to evaluate the effects of mannose on the mRNA expression of β-catenin. Western blotting was conducted to explore the effects of mannose on the ERK/GSK-3β/β-catenin/SNAIL axis and nuclear accumulation of β-catenin. An animal model was established to evaluate the antitumoural effect of mannose on hepatic metastasis in vivo.
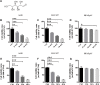
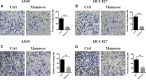
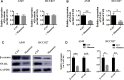
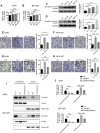
Results: In this study, we found that mannose inhibited the proliferation of A549 and HCC827 cells in vitro both time- and dose-dependently. However, it exerted only a slight influence on the viability of normal lung cells in vitro. Moreover, mannose also inhibited the migrating and invading capacity of NSCLC cells in vitro. Using Western blotting, we observed that mannose reduced SNAIL and β-catenin expression and ERK activation and promoted phospho-GSK-3β expression. The ERK agonist LM22B-10 promoted the metastatic ability of NSCLC cells and increased SNAIL and β-catenin expression in cancer cells, which could be reversed by mannose. Furthermore, ERK-mediated phosphorylation of the β-catenin-Tyr654 residue might participate in the nuclear accumulation of β-catenin and its transcriptional function. The results from animal experiments showed that mannose effectively reduced hepatic metastasis of A549 cells in vivo. Furthermore, mannose inhibited ERK/GSK-3β/β-catenin/SNAIL in tumour tissues obtained from nude mice.
Discussion: Collectively, these findings suggest that mannose exerts anti-metastatic activity against NSCLC by inhibiting the activation of the ERK/GSK-3β/β-catenin/SNAIL axis, which indicates the potential anticancer effects of mannose.
Keywords: mannose; metastasis; non-small-cell lung cancer; β-catenin.
© 2020 Luo et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

