Expert Consensus on Injection Technique and Area-Specific Recommendations for the Hyaluronic Acid Dermal Filler VYC-12L to Treat Fine Cutaneous Lines
- PMID: 32308460
- PMCID: PMC7152505
- DOI: 10.2147/CCID.S239667
Expert Consensus on Injection Technique and Area-Specific Recommendations for the Hyaluronic Acid Dermal Filler VYC-12L to Treat Fine Cutaneous Lines
Abstract
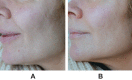
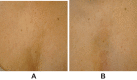
Background: VYC-12L is a hyaluronic acid (HA) injectable gel designed to treat fine cutaneous lines and improve skin quality attributes such as hydration and elasticity.
Objective: Expert consensus was sought on VYC-12L injection technique and primary treatment target areas.
Methods: A multinational group of aesthetic medicine clinicians (n = 128) attended product training and each identified ~10 patients for VYC-12L. After treating their first and last patients, the clinicians completed a survey on preferred injection methodology/technique, including injection angle, volume, and spacing. An expert panel (n = 12) discussed survey results and their clinical experiences to obtain consensus on VYC-12L technique and appropriate treatment areas.
Results: Recommendations included micro-depot injections of VYC-12L into the deep dermis with a 32G ½ inch needle inserted at <45º to the skin, spaced 0.5‒1.0 cm apart, with 0.01‒0.05 mL volume per injection (full-face total volume: ~2 mL). Recommended primary treatment areas were the malar, perioral, neck, and décolletage regions. Injection techniques for different treatment areas/demographic characteristics were similar, with some variability in treatment approach. Patient selection criteria, pre- and post-treatment guidelines, and managing patient expectations are important components of treatment.
Conclusion: These consensus recommendations may assist clinicians in optimizing the treatment of fine lines with VYC-12L.
Keywords: Juvéderm Volite; consensus; dermis; hyaluronic acid; injectable dermal filler; skin aging.
© 2020 Ogilvie et al.
Conflict of interest statement
P Ogilvie is a consultant, trainer, and principal investigator for Allergan plc, participated in an Allergan Juvéderm Volite advisory board, and received research funding for the clinical registration trial of VYC-12L. M Cavallini, J Chantrey, V Figueiredo, I Heydenrych, E Langeland, M Safa, D Sykianakis, J Thulesen, and A Wetter are investigators for Allergan plc and have participated in an Allergan Juvéderm Volite advisory board. J Chantrey received honorarium from Allergan, during the conduct of the study; honorarium for teaching from Allergan, outside the submitted work. V Figueiredo and E Langeland report personal fees, non-financial support from Allergan, outside the submitted work. I Heydenrych reports personal fees from Allergan, during the conduct of the study; personal fees, non-financial support from Allergan, personal fees from SkinCeuticals, outside the submitted work. C Leys is an investigator for Allergan plc, participated in an Allergan Juvéderm Volite advisory board, and received research funding for the clinical registration trial of VYC-12L. J Thulesen is a consultant for Allergan plc, participated in an Allergan Juvéderm Volite advisory board, and is a faculty member of the Allergan Medical Institute. The authors report no other conflicts of interest in this work.
Figures


References
-
- Lai M, Oruc I, Barton JJ. The role of skin texture and facial shape in representations of age and identity. Cortex. 2013;49(1):252–265. - PubMed
LinkOut - more resources
Full Text Sources

