The Paneth Cell: The Curator and Defender of the Immature Small Intestine
- PMID: 32308658
- PMCID: PMC7145889
- DOI: 10.3389/fimmu.2020.00587
The Paneth Cell: The Curator and Defender of the Immature Small Intestine
Abstract
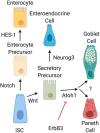
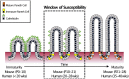
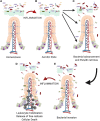
Paneth cells were first described in the late 19th century by Gustav Schwalbe and Josef Paneth as columnar epithelial cells possessing prominent eosinophilic granules in their cytoplasm. Decades later there is continued interest in Paneth cells as they play an integral role in maintaining intestinal homeostasis and modulating the physiology of the small intestine and its associated microbial flora. Paneth cells are highly specialized secretory epithelial cells located in the small intestinal crypts of Lieberkühn. The dense granules produced by Paneth cells contain an abundance of antimicrobial peptides and immunomodulating proteins that function to regulate the composition of the intestinal flora. This in turn plays a significant role in secondary regulation of the host microvasculature, the normal injury and repair mechanisms of the intestinal epithelial layer, and the levels of intestinal inflammation. These critical functions may have even more importance in the immature intestine of premature infants. While Paneth cells begin to develop in the middle of human gestation, they do not become immune competent or reach their adult density until closer to term gestation. This leaves preterm infants deficient in normal Paneth cell biology during the greatest window of susceptibility to develop intestinal pathology such as necrotizing enterocolitis (NEC). As 10% of infants worldwide are currently born prematurely, there is a significant population of infants contending with an inadequate cohort of Paneth cells. Infants who have developed NEC have decreased Paneth cell numbers compared to age-matched controls, and ablation of murine Paneth cells results in a NEC-like phenotype suggesting again that Paneth cell function is critical to homeostasis to the immature intestine. This review will provide an up to date and comprehensive look at Paneth cell ontogeny, the impact Paneth cells have on the host-microbial axis in the immature intestine, and the repercussions of Paneth cell dysfunction or loss on injury and repair mechanisms in the immature gut.
Keywords: cathelicidin (LL37); cell death; defensins; immature intestine; necrotizing enterocolitis; paneth cell.
Copyright © 2020 Lueschow and McElroy.
Figures

References
-
- Paneth J. Ueber die secernirenden Zellen des Dünndarm-Epithels. Arc Mikrosk Anat. (1887) 31:113–91. 10.1007/BF02955706 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

